- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Effect of Ovum Pick-Up Frequency on Quality of Buffalo Oocytes
Sheng QG1*, Tang CM1, Jian H1, Hui L1, Zhun TZ1, Yan YC1, Xiang HF1, Zhuang YB1, Qi YN1, Shun SD2 and Xiang HJ1
1Guangxi Buffalo Research Institute, Chinese Academy of Agricultural Science, Ministry of Agriculture (Guangxi) Key Laboratory of Buffalo Genetics, Breeding and Reproduction, Ministry of Agriculture, China
2State Key Laboratory for Conservation and Utilization of Subtropical Agro-bio resources, Guangxi University, China
*Corresponding author: Sheng QG, Guangxi Buffalo Research Institute, Chinese Academy of Agricultural Science, Ministry of Agriculture (Guangxi) Key Laboratory of Buffalo Genetics, Breeding and Reproduction, Ministry of Agriculture, China
Submission: June 16, 2020;Published: June 29, 2020

ISSN: 2576-9162 Volume7 Issue4
Abstract
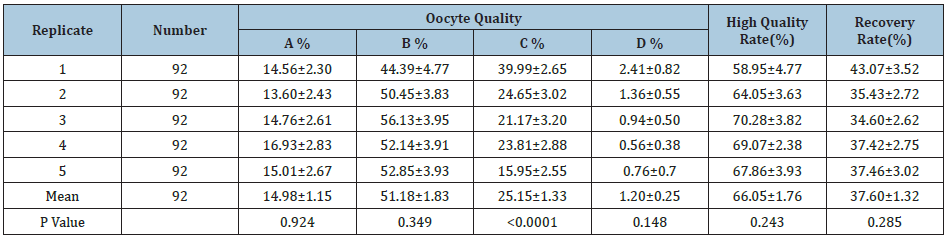
The frequency of live ovum pick-up (LOPU) is one of the main factors affecting buffalo oocyte collection. This study discussed the effect of LOPU frequency on buffalo oocyte collection in purpose of improving the efficiency of water buffalo embryo in vitro production and laying a foundation for the establishment of a high efficient system. 92 buffalo buffaloes from Guangxi Buffalo Research Institute (including Murrah, Nili-Ravi buffalo and hybrid buffaloes), aged 4-12 years, weighing 580-700kg, were used for LOPU for 5 consecutive times at a frequency of every 3 days. The donors were not treated with hormone and the oocytes collected were divided into A, B, C and D grades. All data(the number of visual oocytes ,punctured follicles, collected oocytes, available oocytes, recovery rate, availability rate, follicles, punctured follicles, recovered oocytes, etc.) was collected and counted to obtain the average number of oocytes in LOPU and the rate of high quality oocytes were evaluated. The number of admissible follicles, puncture follicle, collected oocytes were expressed by mean ±standard error (S.E.M). The statistical software SPSS 17.0 was used to analyse the variance. The results showed that the average percentage of high quality oocytes (A and B grade) was 66.05%. When conducting continuous LOPU, the rate of high quality oocytes (grade A) from the first batch to the fifth batch were 58.95%, 64.05% and 70.28%, 69.07% and 67.86%, respectively, while the recoveries rate were 43.07%, 35.43% 4.60%, 37.42% and 37.46%respectively,both showed no significant difference between each batch (P >0.05).However, the rate of grade C oocytes (naked eggs and reticular cells) during 5 times were 39.99%, 24.65%, 15.95%, 23.81% and 21.17%, respectively, showed a significant difference (P<0.0001), and with a decreasing trend as the frequency of LOPU increases. This study laid a foundation for further improving of the efficiency of buffalo LOPU.
Keywords: Buffalo; LOPU frequency; Oocyte quality
Introduction
The domestic bufalo (Bubalus bubalis), a multipurpose livestock species in Asia, provides milk, meat, and draught power .The low fecundity of buffalo is characterized by poor superovulation [1], low recovery of transferable embryos, and low pregnancy rate, as well as some basic physiological characteristics, such as delayed age of puberty, less pronounced signs of estrus, seasonal anoestrus, long period of post-partum anoestrus [2-4]. The Ovum pick-up (OPU) and the in vitro embryo production (IVEP) are currently the most competitive technologies to produce transferable embryos (TE) over the long term [5-8]. Furthermore, OPU can be performed on a wide variety of donors, such as non-cyclic animals, pregnant cows, and subjects with non-patent oviducts or genital tract infections [7,9]. It can also be carried out in animals that do not respond to multiple ovulation procedures, the last representing a high proportion in buffalo species [10]. Therefore, this procedure is a valid alternative to multiple ovulation/embryo transfer (MOET) programs [8], because of the low response to hormonal stimulation, the poor embryo recovery [1,8,11], and the impossibility to repeat continuously the MOET treatments over the long-term. The application of OPU-IVEP technology to buffalo can enhance the maternal contribution to genetic improvement [8,12], thus overcoming the reduced levels of efficiency linked to the reproductive seasonality pattern of this species. This study discussed this factor to improve the efficiency of water buffalo in vitro production technology system and lay a foundation for the establishment of efficient buffalo in vitro production technology system.
Material and Method
If not otherwise stated, all chemicals and reagents were purchased from Sigma l (St. Louis, MO), with the exception of fetal bovine serum (FBS), which was purchased from Gibco BRL (Paisley, Scotland, UK).
Animal and OPUThe study was conducted in a farm located in the veterinary staff of the Division of Animal Resources of Guangxi Buffalo Research Institute (GBRI) Chinese Academy of Agricultural Sciences (CAAS).Ovum pick-up was carried out healthy, multiparous and lactating buffalo cows (Murrah, Nili-Ravi and cross-breed buffalo) with 92 heads. The donors were under controlled nutrition, barnhoused, and restrained in a chute at the moment of the oocyte retrieval session, then prepared for follicular as described by the reporters [5,7,13] for buffaloes. HS2000 Ultrasonic imaging produced by Honda of Japan, with 7.5/5.0MHz vaginal sector scan probe (Aloka, SSD-500, Tokyo, Japan) equipped with 55cm long needle for oocyte collection, vacuum pumps (Cook IVF Co., Australia), follicular fluid collection tube, temperature devices. The number and size of follicles in each ovary was determined before puncture. Follicles of ≥3mm in diameter were punctured and the follicle contents were drawn into the 50-ml Falcon tube using a regulated vacuum pump at 50mmHg vacuum pressure. During follicle aspiration the aspiration line was continuously rinsed with aspiration medium. OPU was conducted once every three days intervals and performed five times continuously.
COCs processingAspiration medium consisted of DPBS with addition of 3% fetal bovine serum (FBS) and 100μg/ml, penicillin and 60mg/ml streptomycin and 10IU/ml heparin. The rinsed TCM199 medium contained 5% NBS. According to the morphological characteristics of cumulus cell complex (COCs), the oocytes were classified according to the method described by Zhang Xiufang et al. [14].
Cumulus-oocytes complexes (COCs) were classified in 4 categories.
- Grade A (oocytes with more than two layers of cumulus cells and homogenous cytoplasm);
- Grade B (oocytes with at least one layer of cumulus cells and homogenous cytoplasm);
- Grade C (oocytes partially denuded, but still showing homogenous cytoplasm);
- Grade D (degenerated oocytes with irregular shrunken cytoplasm). Grade A and B COCs were cultured in vitro maturated medium (TCM199+ 10% FBS+ 10µg/mL FSH + 12 U/mL LH + 1 µg/mL E2+ 50 µM cysteine) under a humidified atmosphere of 5% CO2 at 39°C for 22 to 24 h(Pang et al, 2009)
The average number of oocytes obtained by OPU and the rate of high-quality oocytes were evaluated. SPSS 17.0 software was used for statistical analysis. With different batches as fixed factors, the number of follicles was regarded as dependent variable and the number of follicles was regarded as dependent variable. Single factor analysis of variance (One-Way ANOVA) was used to test significance. The difference was significant by Duncan’s method, using P<0. 05 as the criterion of significance.
Result and Discussion
The results showed that the average percentage of high-quality oocytes (A and B grade) was 66.05%. When conducting continuous LOPU, the rate of high quality oocytes (grade A)from the first OPU to the fifth one were 58.95%, 64.05% and 70.28%, 69.07% and 67.86%, respectively, while the recoveries rate were 43.07%, 35.43% 4.60%, 37.42% and 37.46%respectively,both showed no significant difference between each batch (P>0.05).However, the rate of grade C oocytes (naked eggs and reticular cells) during 5 times were 39.99%, 24.65%, 15.95%, 23.81% and 21.17%, respectively, showed a significant difference (P< 0.0001), and with a decreasing trend as the frequency of LOPU increases.
A literature [15] reported that although there were differences in the average number of punctured follicles and the number of oocytes recovered from every donor successive three times of OPU, there was no difference in the number of oocytes available to each head. In this study, the quality oocytes of grade A and B were the lowest in the first OPU, the second time was increased, the fourth time was relatively stable, but there was no significant difference among the batches of OPU. However, the changes of C grade oocytes were significantly different among batches (P<0.0001). With the increase of the number of OPU, the percentage of C grade oocytes (denuded oocytes and reticular cells) decreased continuously. However, there was no significant difference in the percentage of high quality oocytes and the recovery rate among the batches (P> 0. 05). The average percentage of high quality oocytes of grade A and B grade were 66. 05% and was similar to the result which 70.92 % [15]. Another the recovery rate of oocytes was 71.60% in Murrah buffalo which were treated with eCG [16]. Furthermore, average recovery of no hormonal treatment buffalo oocytes had the similar result (64.18%).
As for the recovery rate of OPU in buffalo, [15] reported that oocyte availability rate in the first OPU was only 56.20%, and the first oocyte availability rate in this study was 58.95%, the results were very similar. But the availability rate of the 2nd and third OPU (64.05±3.63%, 70.28±3.82%) was lower than that of Liang Xianwei (80.98%, 80.60%), which might be different from the variety, quantity and season of the donor, nutrition and other factors related. But the incidence of good quality oocytes (Grade A +B COCs) was also not affected by season in Mediterranean buffaloes [17]. The reason for the low availability of oocytes in the first oocyte collection may be that there are more atresia follicles at the first time than at other times. It was agree with the point of view that higher level of follicular atresia was reported [18] and, consequently, a lower number of total recoverable and viable oocytes. With the increase of the number of oocytes collected the number of the atretic follicles decreases and the quality of the oocytes increases. As a result, the proportion of available COCs recovered increased [19]. The results also showed that the quality oocytes remained relatively stable at the fifth time. However, there was no significant difference in the recovery of oocytes. However, the changes of C-grade oocytes in different batches were significantly different (P< 0.0001). With the increase of the number of OPU, the percentage of C-grade oocytes (denuded oocytes and reticular cells) decreased continuously. The average rate of denuded oocytes recovered in this study was 25.15%, which was higher than that reported by Liang Xianwei et al. [15] (16.33%), but lower than that reported by Yadav et al [16] (39.9%) Figure 1 and Table 1.
Figure 1: Effect of OPU replicate on oocyte quality.
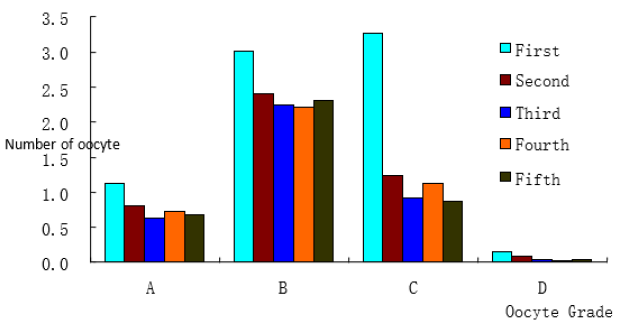
Table 1: Effect of OPU replicate on oocyte quality (Mean±S.E.M).
Conclusion
OPU replicate effected on oocyte quality. With the increase of the number of OPU, the number of high quality oocytes (GradeA+B) increases. There was significant difference in Grade C.
Acknowledgement
This research project is supported by Guangxi key R & D Program (Gui Ke AB 16380040) and Guangxi Science and Technology Development Project (Gui Ke AD19259009) and State Key Laboratory for Conservation and Utilization of Subtropical Agro-bio resources ,Guangxi University (SKLCUSA-b201814).
References
- Zicarelli L (1997) Superovulatory response in buffaloes bred in Italy. Third Course on Biotechnology of Reproduction in buffaloes proceedings Caserta, Italy, pp. 167-188.
- Madan ML (1988) Status of reproduction in female buffalo. II World Buffalo Congress, New Dheli, Compendium of Latest Research, pp. 89-100.
- Singh G (1988) Seasonal trend of calving and subsequent service-period in rural buffaloes in Punjab (India). Acta Vet Stand Suppl 83: 80-84.
- Singla SK, Manik RS, Madan ML (1996) Embryo biotechnologies in buffaloes: A review. Bubalus Bubalis 1: 53-63.
- Boni R, Di Palo R, Barbieri V, Zicarelli L (1994) Ovum pick-up in deep anestrus buffaloes. Proc IV World Buffalo Congress, pp. 480-482.
- Boni R, Roviello S, Zicarelli L (1996) Repeated ovum pick-up in Italian mediterranean buffalo cows. Theriogenology 46(5): 899-909.
- Boni R, (1997) Ovum pick-up in cattle and buffalo. Proc Third Course on Biotechnology of Reproduction in Buffaloes, Issue II, Caserta, Italy, pp. 93-104.
- Gasparrini B (2002) In vitro embryo production in buffalo species: State of the art. Theriogenology 57(1): 237-256.
- Galli C, Crotti G, Notari C, Turini P, Duchi R, Lazzari G (2001) Embryo production by ovum pick-up from live donors. Theriogenology 55(6): 1341-1357.
- Karaivanov C (1986) Comparative studies on the superovulatory effect of PMSG and FSH in the water buffalo (Bubalus Bubalis). Theriogenology 26(1): 51-59.
- Zicarelli L (1994) Anaestro and induction of estrus in acyclic buffaloes. Agriculture and Research 153: 55-81.
- Gasparrini B (2010) Test-tube fertilization in buffalo. Pre-Congress courses in biotechnology of the buffalo reproduction. comparison with the cow. Proceedings of the 9th World Buffalo Congress Buenos Aires, Revista Veterinaria, pp. 80-98.
- Guangsheng Q, Xianwei L, Mingtang C, Zhongquan L (2005) Milk buffalo oocytes were collected in vivo by B mode ultrasound. Cattle Journal 31(2): 28-29.
- Xiufang Z, Xianwei L, Mingtong C, Chunying P, Finxiang H, et al (2007) Studies on in vitro fertilization and embryonic development of buffalo oocytes. Animal Husbandry and Veterinary Medicine 39(11): 15-17.
- Xianwei L, Xiufang Z, Bingzhuang Y, Mingtang C, Fenxiang H, et al (2008) In Vitro embryo production in Vitro from Buffalo in Vitro. Chinese Journal of Veterinary Medicine 28(10): 1229-1232.
- Yadav SK, Misra AK, Sharma R, Prasad Shiv, Gupta HP (2006) Repeated trans-vaginal ultrasound guided aspiration of oocytes (OPU) from murrah buffaloes. Proceedings of the 5th Asia Buffalo Congress, Nanning, China, pp. 621-627.
- Francesco D, Novoa S, Vecchio MV, Neglia D, Boccia G, et al (2012) Ovum pick-up and in vitro embryo production (OPU-IVEP) in Mediterranean Italian buffalo performed in different seasons. Theriogenology 77(1): 148-154.
- Van Ty L, Chupin D, Draincourt MA (1989) Ovarian follicular populations in buffaloes and cows. Anim Repr Sci 19(3-4): 171-178.
- Campanile G, Baruselli PS, Neglia G, Vecchio D, Gasparrini B, et al (2010) Ovarian function in the buffalo and implications for embryo development and assisted reproduction. Anim Reprod Sci 121(1-2): 1-11.
© 2020 Sheng QG. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






