- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Isolation, Serological and Molecular Detection of Infectious Laryngotracheitis Virus (ILTV) in Chickens in Libya
Abdulwahab Kammon1*, Jamaal Shabba1 and Yousef Abouzeed2, and Abdulatif Asheg3
1Department of Poultry and Fish Diseases, University of Tripoli, Libya
2Department of Microbiology, Faculty of Veterinary Medicine, University of Tripoli, Libya
3University of Subratha, Libya
*Corresponding author: Abdulwahab Kammon, Department of Poultry and Fish Diseases, Libya
Submission: January 27, 2020;Published: February 07, 2020

ISSN: 2576-9162 Volume7 Issue3
Abstract
Infectious Laryngotracheitis (ILT) is a viral respiratory disease of poultry caused by Gallid herpesvirus 1 leading to severe economic losses. The aim of the current study was to isolate and identify ILT virus in suspected outbreaks of one broiler flock (farm 1) and three layer flocks (farms 2,3 and 4) located in Tripoli. Samples of trachea were collected and preserved in transport media for viral isolation and some were preserved in 10% neutral formalin for histopathology. Broiler and Layer flocks were serologically monitored and tested for the presence of antibodies by ELISA against ILTV at first days old and later at slaughter age for broilers and before the onset of production for layers in which total of 290 blood samples were collected from six poultry farms (3 broiler flocks and 3 layer flocks). Following isolation of the virus in fertile eggs, chorio-allantoic membrane (CAM) samples were confirmed by PCR and histopathology. The lesions seen on the CAMs were congestion, hemorrhages and pocks after 5 days post inoculation. Samples from farm 1 and 3 were positive whereas other farms were negative. Histopathology of CAMs revealed edema, congestion, haemorrhages, infiltration of heterophils and mononuclear cells, and presence of syncytial cells containing intranuclear inclusion bodies. The trachea revealed severe destruction and sloughing of the mucosa due to necrosis. The results of this study confirmed the circulation of ILTV in commercial poultry flocks and highlighted the need for more epidemiologic investigation of the disease in the whole country. The next step will be the phylogenetic analysis of the viral genome by sequencing
Keywords: ILT; Broilers; Layer; PCR; Virus isolation; Histopathology; ELISA
Introduction
Infectious Laryngotracheitis (ILT) is a viral respiratory disease of poultry. ILT is classified as a member of the family Herpesviridae in the subfamily Alphaherpesvirinae. The virus is taxonomically identified as Gallid herpesvirus 1 [1]. Generally, Herpesviruses produce pathogenically important proteins such as envelop, tegument, capsid, glycoproteins including gL, gM, gH, gB, gC, gK, gG, gJ, gD, gI, gE, thymidine kinase (TK), transcriptional regulator and even non-structural proteins. gG is a conserved molecule among most alpha Herpesviruses that binds to and modulates the biological activity of chemokines [2,3]. Avian infectious laryngotracheitis (ILT) is a major viral respiratory disease which is included within List B of the Office International des Epizooties [4]. The disease was first described in 1925 by May and Thittsler [5]. Geographically, ILT was described in many countries in areas of intensive poultry production of chicken such as North America, South America, Europe, China, South East Asia and Australia. Laboratory diagnosis is required for ILT, because other diseases cause similar clinical signs and lesions, such as infectious bronchitis, Newcastle disease, avian influenza, infectious coryza, and mycoplasmas. ILTV infection can be confirmed using several methods, including virus isolation and DNA detection. A commercial ELISA for detection of antibodies against ILTV is also available. For ILTV isolation, the CAM inoculation of 9-to-12-d-old embryos is used. Swab or organ samples from the trachea, conjunctiva, larynx, and lung of clinically affected birds are collected and inoculated on the CAM. Multinucleated giant cells and intranuclear inclusions may be observed 24 hours post infection [6]. ILT DNA detection methods have developed rapidly in recent years. These methods can identify ILTV quickly, accurately, and are highly sensitive. Molecular techniques for ILTV detection include cloned DNA probes for dot blot hybridization, PCR, nested PCR, real-time PCR, multiplex PCR, in situ hybridization [7,8], and PCR followed by restriction fragment length polymorphism (RFLP) [9]. ILTV detection with PCR was more sensitive than virus isolation in cell culture and electron microscopy. Several highly conserved genes within the herpes viruses were selected for establishment of real-time PCR in previous studies, such as gG and TK genes [10-12]. Compared with electron microscopy, histologic test, viral isolation via CAM route, FA test, and real-time PCR for ILT diagnosis in a broiler farm outbreak, the real-time PCR was the most sensitive method for ILTV detection. Since many laboratories are not set up for real-time PCR; FA, histopathology, and conventional PCR are the most commonly methods used for routine diagnosis of ILT [13].
In Libya, vaccination against ILT is not permitted by the Veterinary Authority (National Centre of Animal Health). Few studies were previously conducted for diagnosis of field infections of ILT. Asheg et al. [14] reported an outbreak of ILT in layer chickens diagnosed by ELISA and presence of intranuclear inclusion bodies. Later on, a partial sequencing of glycoprotein G gene of Gallid herpesvirus 1 strain ILT-1, was reported to GenBank by Asheg in 2013 (unpublished data). Phylogenetic analysis was not previously carried out to compare this strain with other regional or international strains including the vaccine strain. Moreover, there is a lack of information about the presence of antibodies against ILT in chickens and some ILT outbreaks occurred from time to time where the causative virus was not isolated or molecularly characterized. Therefore this study is reporting isolation and molecular identification of ILTV during a field outbreak. Moreover, monitoring of antibodies against ILTV in some commercial layers and broiler flocks were conducted.
Material and Method
Samples of trachea collected from diseased chickens during four outbreaks of suspected ILT infections were preserved in transport media contain antibiotics and antifungal drugs and transferred to the Laboratory of the Department of Poultry and Fish Diseases. All information about the outbreaks including location, total number of birds in each flock, poultry species and mortality are shown in Table 1.
Table 1: Case history of ILT suspected cases.
Inoculation of chicken fertile eggs on chorioallantoic membrane (CAM)
The preserved field samples were thawed and macerated using sterilized pestle and mortar to prepare a 10-20% (w/v) suspension in sterile PBS. The suspension was then centrifuged at 3000rpm for 30 minute for clarification. Supernatant fluid was treated with broad-spectrum antibiotics and antifungal (Gentamycin 50μg/ml, penicillin 2000 units/ml, streptomycin 2mg/ml and mycostatin 1000 units/ml) at room temperature for one hour. The sterility of the inoculum was checked on blood agar. The resulting suspension was centrifuged at low speed to remove debris, and 0.1ml of the supernatant fluid was inoculated on the dropped CAM of at least three embryonated chicken eggs of 10-12 days’ of incubation. The eggs were sealed with paraffin wax, incubated at 37 °C for up to 7 days and candled daily. The CAMs of dead embryos or of those surviving for 7 days were examined for the presence of typical pocks and samples were preserved in 10% buffered neutral formalin for histopathology.
Identification by histopathologySegments of trachea of diseased birds and CAMs following inoculation were preserved in 10% buffered neutral formalin. After clarification, samples were stained with hematoxylin and eosin as per the method previously described [15]. Slides were screened under light microscope with different power (10X, 40X and 100X after putting immersion oil). Intra-nuclear inclusion bodies and syncytial cell formations in trachea and CAMs epithelium if any, were recorded and photographs were taken.
Extraction of samples DNA and PCR analysisTissue samples (trachea and CAMs) were cut into small pieces and 5% homogenates were prepared in phosphate buffered saline (pH 7.2). DNA was extracted using Wizard® Genomic DNA Purification Kit (Promega, USA) as per the manufacturer’s instructions. The PCR was performed with a set of ILTV specific primers amplifying a 647-bp fragment as previously described [16].
SerologyBroiler and layer flocks were monitored and tested for the presence of antibodies against ILTV at first days of old and later on at slaughter age for broilers and before the onset of production for layers. A total of 290 blood samples were collected from six poultry farms (3 broiler flocks and 3 layer flocks located in Tripoli, Libya). Blood samples were withdrawn from each flock at the 3 days old (Table 2). Another set of samples were collected from the same flocks at the slaughter time and before the point of lay for broilers and layers, respectively.
Table 2: Blood samples of broiler and layer flocks at different ages.
Serological analysis using Enzyme linked immunosorbent assay (ELISA)
The ILT ELISA kit was obtained from BioChek Ltd (London, UK). The ILT ELISA kit measures the amount of antibody to the Gallid herpesvirus 1 in the serum of chickens. The analysis was performed as per the manufacturer’s instructions. Microtitre plates have been pre-coated with inactivated ILTV antigen. Chicken serum samples were diluted and added to the microtitre wells where any anti- ILT antibodies present will bind and form an antigen-antibody complex. Non-specific antibodies and other serum proteins were then washed away. Anti-chicken IgG labeled with the enzyme alkaline phosphatase was then added to the wells and binds to any chicken anti-ILT antibodies originally bound to the antigen. After another wash to remove unreacted conjugate, substrate was added in the form of pNPP chromogen. A yellow color was developed if anti-ILT antibody is present and the intensity is directly related to the amount of anti-ILT present in the sample.
Statistical analysisData were expressed as mean ± standard error. The differences of antibody levels (S/P ratios) in broilers of the same flock at 3 days and at slaughtering age were compared by T test using SPSS software (SPSS Inc. Chicago, Illions, USA). The values of (P≤0.05) were taken as the cut-off value to consider differences that are statistically significant.
Result
Suspected ILT cases were submitted to the Department of Poultry and Fish Diseases for diagnosis (Table 1). Necropsies were conducted and appropriate samples were collected for confirmation. Suspected cases included:
Case 1: Live and Dead broiler chickens of 45 days-old belonging to a broiler farm located in Gaser bin Gishir. The live birds showed respiratory clinical signs characterized by depression, gasping and expectoration of bloody mucus, sinusitis, and conjunctivitis. The mortality was very high accompanied with consequent retarded growth. Postmortem (PM) lesions indicated clear infection of upper respiratory tract with hemorrhage and caseous material in the trachea, conjunctivitis, sinusitis and mucoid tracheitis.
Case 2: Live and Dead chickens aged 24 weeks old brought from commercial Layers farm in Gaser bin Gishir. The clinical signs included respiratory infection coughing, gasping, nasal discharges, watery eyes swelling of infraorbital sinuses, emaciation, and drop in egg production. PM lesions included presence of mucus, and yellow caseous exudates in the lumen of trachea with tracheal congestion.
Case 3: Dead layer chickens aged 30 weeks old brought from Layers farm in Gaser bin Gishir. According to the owner, the clinical signs included coughing, gasping, rattling, and extension of the neck, sinusitis, conjunctivitis and low mortality. There was severe reduction in egg production. PM lesions included presence of blood, mucus, yellow caseous exudates in the lumen of trachea.
Case 4: Live and Dead chickens aged 24 weeks old brought from Layers farm in Gaser bin Gishir. The clinical signs included coughing, gasping, nasal discharge, emaciation, and drop in egg production. PM lesions included congestion in larynx and trachea.
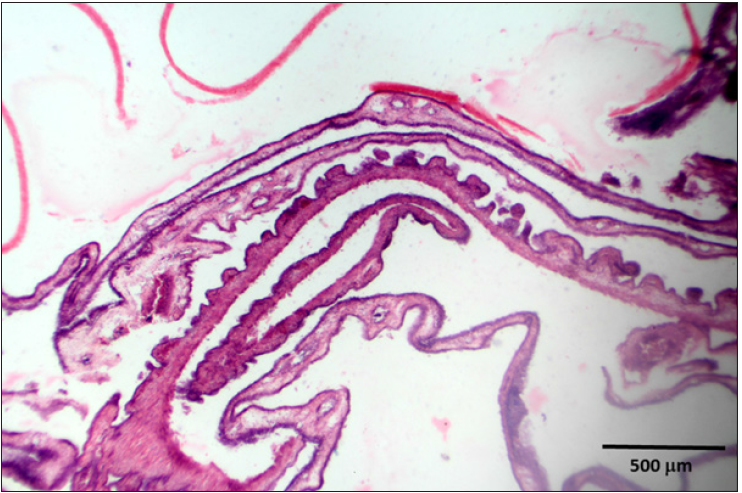
Viral isolation and identification in fertile eggsILTV was isolated on chorioallantoic membrane (CAM). Inoculation of chicken fertile eggs on CAM with samples from cases 1 and 3 revealed presence of congestion, hemorrhages and pock lesions after 5 days post inoculation (Figure 1). Samples from other cases were negative (Figure 2).
Figure 1: CAM showing congestion, hemorrhages and pock lesions after 5 days post inoculation.

Figure 2: Normal chorioallantoic membrane (CAM).
Histopathology
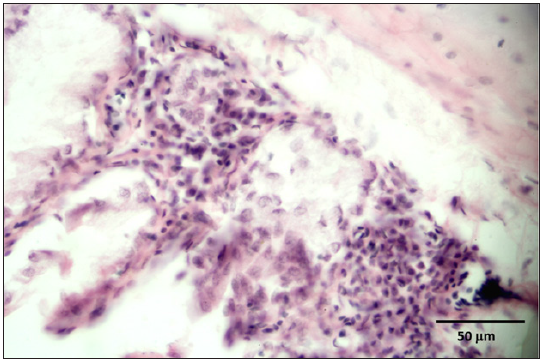
Stained slides from trachea and CAMs were screened under the light microscope. CAMs collected from cases 1 and 3 were congested, hemorrhagic and edematous (Figure 3) as compared to normal (Figure 2). The CAMs collected from cases 1 and 3 were heavily infiltrated with lymphocytes, mononuclear cells (Figure 4 & 5) and many of heterophils with congestion and haemorrhages (Figure 6 & 7). Numerous syncytial cells were also presence in CAMs with intranuclear inclusion bodies in these cells (Figure 8, 9 & 10). Some cells showed presence of intranuclear inclusion body pressing the nucleic material to the periphery of the nucleus (Figure 11). Tracheal samples showed sloughing of the mucosa of the trachea with inflammation (Figure 12) with destruction of the tracheal mucosa due to necrosis and presence of intra-nuclear inclusions within a syncytial cell (Figure 13).
Figure 3: CAM showing congestion, haemorrhages and edema.

Figure 4: CAM showing edema and infiltration of lymphocytes and mononuclear cells.
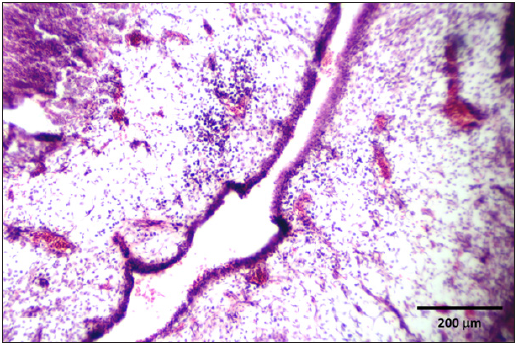
Figure 5: High power of figure 4.
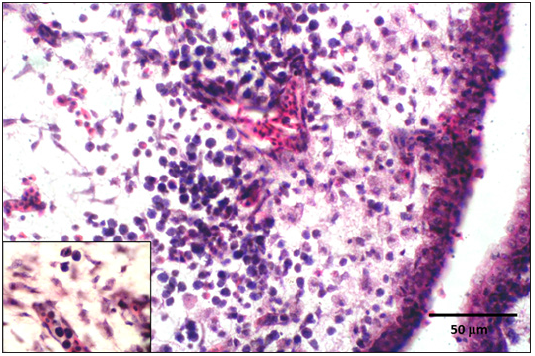
Figure 6: CAM showing infiltration of heterophils with congestion and haemorrhages.s
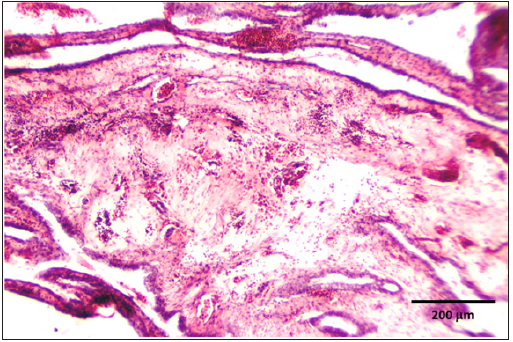
Figure 7: High power of figure 6.
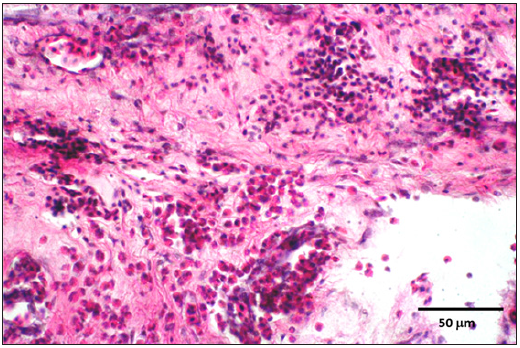
Figure 8: CAM showing syncytial cells containing intranuclear inclusion bodies (inlet).
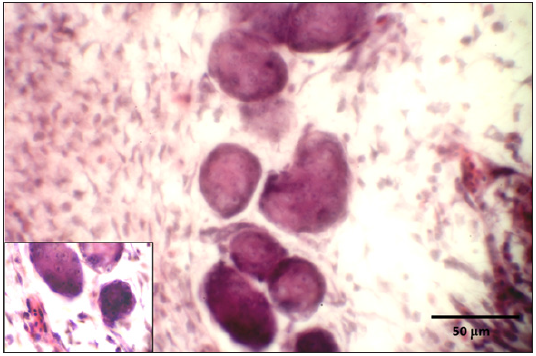
Figure 9: Syncytial cells containing intranuclear inclusions.
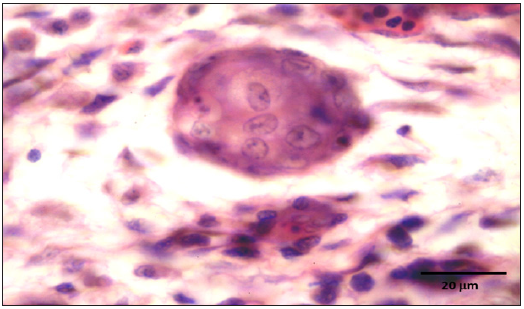
Figure 10: Many syncytial cells showing presence of intranuclear inclusion bodies.
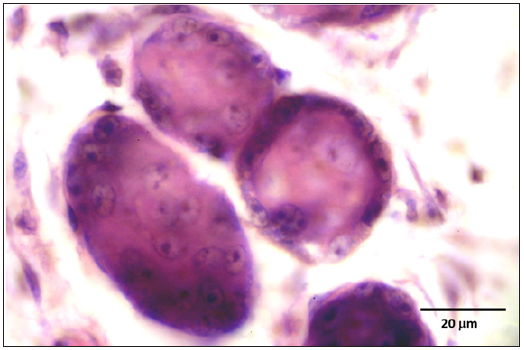
Figure 11: A cell showing intranuclear inclusion body pressing the nucleolus and nucleic material to the periphery of the nucleus (arrow).
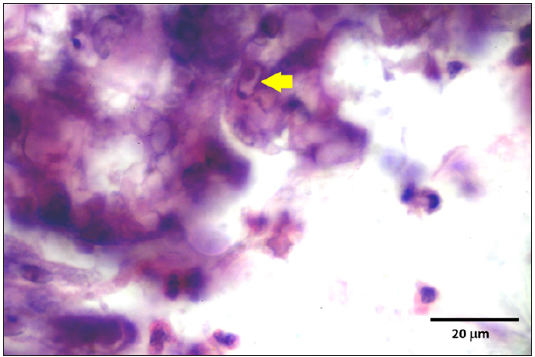
Figure 12: Sloughing of the mucosa of the trachea with inflammation.
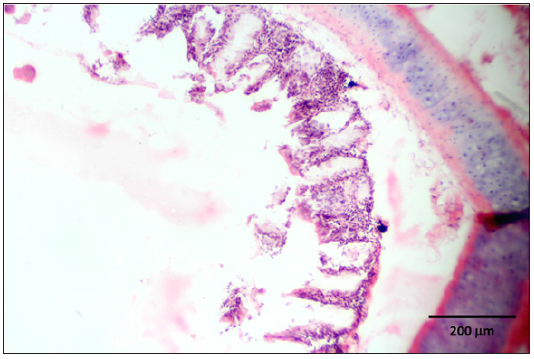
Figure 13: Destruction of the tracheal mucosa due to necrosis and presence of intranuclear inclusions within a syncytial cell.
Polymerase chain reaction (PCR)
Cases 1 and 3 were positive for ILT by PCR. The ILTV was isolated from birds of the same cases.
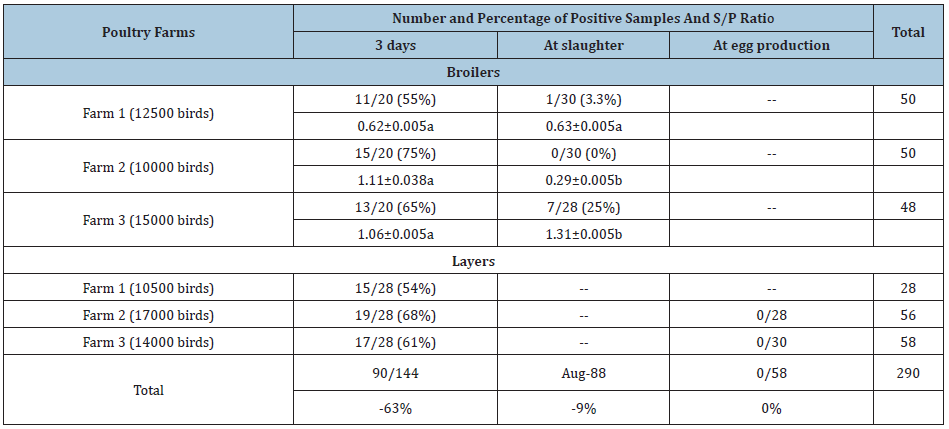
ELISA: In the current study broiler and layer flocks (3 farms each) were tested by ELISA to determine the percentage of positive samples for ILT. The total number of tested samples was 290. Results are shown in Table 3. In broilers at 3 days of age, 11 samples out of 20 (11/20) (55%), 15/20 (75%) and 13/20 (65%) were positive for the presence of antibodies against ILT in farm 1, farm 2 and farm 3, respectively. In broilers at slaughtering age, 1 samples out of 30 (1/30) (3.3%), 0/30 (0%) and 7/28 (25%) were positive for the presence of antibodies against ILT in farm 1, farm 2, and farm 3, respectively (Table 3). Mean S/P ratios of antibodies against ILT at 3 days of age were 0.62, 1.11 and 1.01 for broiler farms 1, 2 and 3, respectively. At slaughtering age, the mean S/P ratios were 0.63, 0.29 and 1.31 for farm 1, 2 and 3, respectively. It is an interesting finding that the level of antibodies of chickens in farm 2 have significantly (P≤0.05) declined at slaughtering age as statistically compared with the level of antibodies measured at 3 days old. However, in farm 1 and 3, the level of antibodies were not declined. Moreover, there was a significant (P≤0.05) increase of antibodies level in chickens of farm 3 at slaughtering age as compared with the level of antibodies at 3 days old. In layers at 3 days of age, 15 samples out of 28 (15/28) (54%), 19/28 (68%) and 17/28 (61%) were positive for the presence of antibodies against ILT in farm 1, farm 2, and farm 3, respectively. However, at production age, no antibodies were detected by ELISA (Table 3).
Table 3: Number and percentage of positive samples of broiler and layer flocks tested by ELISA.
Discussion
ILTV causes an acute and chronic respiratory disease in poultry leading to significant economic losses to the poultry industry. The virus is not easy to control, because it is highly contagious, can cause latent infections, and has many transmission sources. Suspected ILT cases derived from Gasir Bin Gashir, the most intensive area of poultry production in Tripoli were submitted to the Department of Poultry and Fish Diseases for diagnosis. Necropsies were conducted and appropriate samples were collected for confirmation. Clinical and pathologic findings were consistent with previously reported severe form of infectious laryngotracheitis. This form is characterized by severe dyspnea, high mortality and ma¬rked hemorrhagic and/or diphtheritic laryngotracheitis and are attributed to wild-type ILTV [17]. Most birds in that report showed typical lesions in the sinus, larynx and the trachea, although the con¬junctiva and lungs were less affected. The gross lesions recorded in the current study were similar to those reported by Calnek et al. [18]. Infection was determined after inoculation of ILTV suspected field samples through CAM route. The typical gross pathological lesions were visually observed as discrete pock formation with opaque edges and central depressed area of necrosis in the CAM and stunting growth of the ILTV affected embryo after 2 days post inoculation four passages. Burnet [19] stated that embryo mortality due to ILTV occurs within 2-12 days post inoculation, and this agree with our result in this study. Asheg et al. [14], described the clinical signs and lesion observed during ILT outbreak in layer chickens in Libya. The clinical signs were included moist rales, coughing and gasping. After 5 days, the clinical signs progressed to marked dyspnea with coughing of blood-stained mucous. The clinical signs spontaneously disappeared, and a complete recovery was observed on days 10. Gross lesions reported by Asheg et al. [14] included haemorrhage and accumulation of mucous in the lumen of trachea. Microscopically, syncytial formation and intranuclear inclusion bodies along with mucous degeneration and necrosis were observed in the tracheal epithelium. The outbreaks diagnosed in the current study may be caused by the same virus reported by Asheg et al. [14]. It is well known that ILTV produces latent infection. ILTV is usually present in tracheal tissues and secretions for 6-8 days post inoculation (PI) [20,21] followed by extra tracheal spread of ILTV to trigeminal ganglia from eye via neural pathway and this was first reported by Bagust et al. [22]. However, confirmation of such hypothesis could be achieved by sequencing of viral DNA and phylogenetic analysis. These facilities are not available at our Department during conduction of this study. Moreover, there was a lack of research materials for sequencing.
The diagnosis by histopathology is considered a valid and relatively rapid test for ILT [23]. Multinucleated cells (Syncytia) formation and intranuclear inclusion bodies are considered pathognomonic lesions for the diagnosis of ILT [24-27]. Microscopic changes vary according to the stage of the disease. Early microscopic changes in tracheal mucosa include the loss of goblet cells and infiltration of the mucosa with inflammatory cells. As the viral infection progresses, cells enlarge, lose cilia, and become edematous. Syncytial cells are formed and lymphocytes, histiocytes and plasma cells migrate into the mucosa and submucosa. Later, cell destruction and desquamation result in a mucosal surface either covered by a thin layer of basal cells or lacking any epithelial covering. Inclusion bodies are generally present for a few days at the early stage of infection before epithelial cells die [28], but they may be present for only 3-5 days after infection. In severe cases, most infected cells are detached from the tracheal lining [6]. In the current study, intranuclear inclusions were observed in tracheal epithelium along with necrosis and sloughing of the cells. These lesions were absent in some tracheal samples. This may be due to the delay of sample collection from trachea for histopathology. These kinds of lesions are observed in many other respiratory diseases, especially infectious bronchitis, avian influenza and Newcastle disease. In this way, lesions induced by ILT virus, such as hypertrophy and hyperplasia of goblet cells, are similar to those observed following infection with velogenic viscerotrope strains of Newcastle disease virus [29]. Besides, differential diagnosis might be complicated by high frequency of bacterial secondary infections. In the current study, histopathology revealed lesions of ILT in 2 out of 4 outbreaks of suspected ILT infections with suspicion of two other respiratory diseases (infectious bronchitis and Newcastle disease). However, we have not attempted to isolate and diagnose other viral pathogens that may accompany ILTV infection. Samples collected from chickens of the cases 1 and 3 had edema, hemorrhages and syncytia with intranuclear inclusion bodies associated with the chorioallantoic membrane (CAM) allowing confirmatory histopathology diagnosis. Hemorrhage may occur in cases of severe epithelial destruction and desquamation and edematous changes was observed with exposure and rupture of blood capillaries [17].
ILTV was successfully isolated in CAM of fertile eggs inoculated with samples belong to case 1 and 3. Srinivasan et al. [30] and Hughes et al. [31] have reported that ILTV can be isolated in embryonic eggs inoculated via the chorioallantoic membrane (CAM) route. Isolation of ILTV from preserved samples from the tracheas of clinically affected birds is conducted by inoculation of embryonated eggs [6]. The isolation of ILTV in this study was confirmed by real time PCR (rPCR). Complementary tests are very important for the demonstration of the etiology of unspecific conjunctivitis and/or tracheitis cases. This condition is described in mild forms of laryngotracheitis related to ILTV vaccine strains [32,33]. In cases where the typical lesions are missing, ancillary diagnostic tests are strongly recommended, such as PCR for definitive diagnosis. In the current study, PCR was used to test clinical samples already analyzed or not by histopathological technique. Cases 1 and 3 were positive for ILT by PCR. The ILTV was isolated from birds of the same cases. The correlation between clinical signs, histopathology features and PCR results demonstrates the importance of the molecular analysis as more rapid tool for ILT diagnosis [34]. Indeed, the PCR technique is also more sensitive than virus isolation and histopathology analyses [26], especially when other contaminant viruses (e.g. Adenovirus) [35] or bacteria (e.g.: Mycoplasma gallisepticum and Mycoplasm synoviae) [36] are present in tested samples. The target gene in this study was gG ILTV which is a partial sequence of glycoprotein G gene of Gallid herpesvirus 1 strain ILT-1, and previously amplified by PCR, sequenced and reported to GenBank by Asheg [14] (unpublished data). At this point, very few reports are available on the existence of the ILTV in Libyan poultry population, and this is the second description on the molecular detection and confirmation of ILT virus (GaHV-1). The viral molecular characterization will provide better comprehension of the potential virulence of ILTV strains, and the relationship between isolates from this outbreak and isolates from other field or vaccine strains.
ELISAs have been described for the detection of antibody to ILT virus in chickens [37-39]. In the current study, three broiler flocks were monitored for the presence of antibodies against ILTV. Samples were collected at 3 days of age and at slaughtering age of each flock. High percentages and high titers of antibodies were detected at early age (3 days) of all the flocks which may indicate vaccination (maternal immunity) or infection of their parents. Although, percentage was declined but the titer of antibodies was still high at the slaughtering age which may attributed to field infection since the maternal immunity usually decline with an increase of the age. Gharaibeh and Mahmoud [40] reported that all maternal antibody titers were depleted by 10d of age for avian encephalomyelitis (AEV), avian influenza (AIV), chicken infectious anemia (CAV), infectious bronchitis (IBV), infectious laryngotracheitis (ILTV), Mycoplasma gallisepticum (MG), Mycoplasma synoviae (MS) and Newcastle disease (NDV) except for IBDV. Same results were found in layer flocks in which samples collected at 3 days old were found positive for antibodies against ILTV. This may also indicate vaccination or infection of parent flocks. However, no antibodies were detected at the age of egg production.
References
- Roizman B (1982) The family herpesviridae: General description, taxonomy and classification. In: Roizman B (Ed.), The Herpesviruses, l I Plenum Press, New York, pp. 1-23.
- Bryant NA, Davis Poynter N, Vanderplasschen A, Alcami A (2003) Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO J 22: 833-846.
- Viejo Borbolla A, Munoz A, Tabares E, Alcami A (2009) Glycoprotein G from pseudorabies virus binds to chemokines with high affinity and inhibits their function. J Gen Virol 91(1): 23-31.
- Office International des Epizooties (OIE) (1999) International Animal Health Code: Mammals, Birds And Bees, 8th edn, Paris, pp. 468.
- Hidalgo H (2003) Infectious laryngotrachetis: A review. Brazialan J Poult Sci 5(3): 157-168.
- Islam MS, Khan MSR, Islam MA, Hassan J, Affroze S, et al. (2010) Isolation and characterization of infectious laryngotracheitis virus in layer chickens. Bangl J Vet Med 8(2): 123-130.
- Nagy E (1992) Detection of infectious laryngotracheitis virus infected cells with cloned DNA probes. Can J Vet Res 56(1): 34-40.
- Nielsen OL, Handberg KJ, Jørgensen PH (1998) In situ hybridization for the detection of infectious laryngotracheitis virus in sections of trachea from experimentally infected chickens. Acta Vet Scand 39(4): 415-421.
- Oldoni I, Rodríguez Avila A, Riblet S, García M (2008) Characterization of infectious laryngotracheitis virus (ILTV) isolates from commercial poultry by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). Avian Dis 52(1): 59-63.
- Abbas F, Andreasen JR, Baker RJ, Mattson DE, Guy JS (1996) Characterization of monoclonal antibodies against infections laryngotracheitis virus. Avian Dis 40(1): 49-55.
- Chang PC, Lee YL, Shien JH, Shieh HK (1997) Rapid differentiation of vaccine strains and field isolates of infectious laryngotracheitis virus by restriction fragment length polymorphism of PCR products. J Virol Methods 66(2): 179-186.
- Kirkpatrick NC, Mahmoudian A, O Rourke D, Noormohammadi AH (2006) Differentiation of infectious laryngotracheitis virus isolates by restriction fragment length polymorphic analysis of polymerase chain reaction products amplified from multiple genes. Avian Dis 50(1): 28-34.
- Crespo R, Woolcock PR, Chin RP, Shivaprasad HL, García M (2007) Comparison of diagnostics techniques in an outbreak of infectious laryngotracheitis from meat chickens. Avian Dis 51(4): 858-862.
- Asheg A, Tarhuni OA, Al Garib SO, Hamid MA, Kammon AM (2011) An outbreak of infection laryngotracheitis in layer chickens in Libya. Indian Vet J 88(8): 135-136.
- Luna LG (1968) Manual of histological staining methods of armed forces institute of pathology. Mc Graw Hill book Co, New York.
- Pang Y, Wang H, Girshick T, Xie Z, Khan MI (2002). Development and application of a multiplex polymerase chain reaction for avian respiratory agents. Avian Diseases 46(3): 691-699.
- Guy JS, Barnes HJ, Smith LG (1990) Virulence of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates. Avian Dis 34(1): 106-113.
- Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM (1997) Diseases of Poultry. 10th edn, pp. 529-530.
- Burnet F (1934) The propagation of the virus of infectious laryngotracheitis on the CAM of developing egg. Br J Ex Pathol 12: 179-198.
- Purcell DA, McFerran JB (1969) Influence of method of infection on the pathogenesis of infectious laryngotracheitis. J Comp Pathol 79(3): 285-291.
- Hitchner SB, Fabricant J, Bagust TJ (1977) A fluorescent-antibody study of the pathogenesis of infectious laryngotracheitis. Avian Dis 21(2): 185-194.
- Bagust TJ (1986) Laryngotracheitis (Gallid-1) herpesvirus infection in the chicken. 4. Latency establishment by wild and vaccine strains of ILT virus. Avian Pathol 15(3): 581-595.
- Office International des Epizooties OIE (2014) Avian Laryngotracheitis, version adopted by the World Assembly of Delegates.
- Purcell DA (1971) Histopathology of infectious laryngotracheitis in fowl infected by an aerosol. J Comp Pathol 81(3): 421-431.
- Guy JS, Barnes HJ, Smith LG (1992) Rapid diagnosis of infectious laryngotracheitis using a monoclonal antibody-based immunoperoxidase procedure. Avian Pathol 21(1): 77-86.
- Williams RA, Bennett M, Bradbury JM, Gaskell RM, Jones RC, et al. (1992) Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction. J Gen Virol 73(9): 2415-2420.
- Guy JS, Bagust TJ (2003) Laryngotracheitis. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, et al. (Eds.), Diseases of poultry, Iowa State Press, USA, pp. 121-134.
- Nair V, Jones RC, Gough RE (2008) Herpesviridae. In: Pattison M, McMullin PF, Bradbury JM, Alexander DJ (Eds.), Poultry diseases. 6th edn, Sunders Elsevier, Philadelphia, USA, pp. 267-271.
- Lai MC, Ibrahim AL (1983) Scanning electron microscopy of tracheal epithelium of chickens infected with velogenic viscerotropic Newcastle disease virus. Avian Dis 27(2): 393-404.
- Srinivasan VA, Mallick BB (1977) Studies on multiple inoculations of infectious laryngotracheitis virus in chickens. Indian Vet J 54: 1-5.
- Hughes CS, Jones RC (1988) Comparison of cultural methods for primary isolation of infectious laryngotracheitis virus from field material. Avian Pathol 17(2): 295-303.
- Sellers H, Garcia M, Glisson J, Brown T, Sander J, et al. (2004) Mild infectious Laryngotracheitis in broilers in the Southeast. Avian Dis 48(2): 430-436.
- Dufour Zavala L (2008) Epizootiology of infectious laryngotracheitis and presentation of an industry control program. Avian Dis. 52(1): 1-7.
- Gowthaman V, Singh SD, Dhama K, Barathidasan R, Mathapati BS, et al. (2014) Molecular detection and characterization of infectious laryngotracheitis (Gallid herpesvirus-1) from clinical samples of commercial poultry flocks in India. Avian Dis 25(3): 345-349.
- Alexander HS, Nagy E (1997) Polymerase chain reaction to detect infectious laryngotracheitis virus in conjunctival swabs from experimentally infected chickens. Avian Dis 41(3): 646-653.
- Couto RM, VilarinhoBraga JF, Sandra YM Gomes, Mauricio R, Nelson RS Martins, et al. (2016) Natural concurrent infections associated with infectious laryngotracheitis in layer chickens. Journal of Applied Poultry Research 25 (1): 113-128.
- Meulemans G, Halen P (1982) Enzyme-linked immunosorbent assay (ELISA) for detecting infectious laryngotracheitis viral antibodies in chicken serum. Avian Pathol 11(3): 361-368.
- York JJ, Fahey KJ, Bagust TJ (1983) Development and evaluation of an ELISA for the detection of antibody to infectious laryngotracheitis virus in chickens. Avian Dis 27(2): 409-421.
- Adair BM, Todd D, McKillop ER, Burns K (1985) Comparison of serological tests for detection of antibodies to infectious laryngotracheitis virus. Avian Pathol 14(4): 461-469.
- Gharaibeh S, Mahmoud K (2013) Decay of maternal antibodies in broiler chickens. Poult Sci 92(9): 2333-2336.
© 2020 Abdulwahab Kammon. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






