- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Reaction of Immune Cells to Campylobacter Jejuni in Chicken PBMC Treated by Different Probiotic Bacteria In Vitro
Viera Revajová1*, Viera Karaffová1, Mária Levkutová2, Miroslava Šefcová1, Andrea Lauková3 and Mikuláš Levkut1,4
1Institute of Pathological Anatomy, Slovakia
2 Institute of Epizootology and Preventive Veterinary Medicine, Košice, Slovakia
3Institute of Animal Physiology, Košice, Slovakia
4Institute of Neuroimmunology, Slovakia
*Corresponding author: Viera Revajová, Institute of Pathological Anatomy, Slovakia
Submission: January 27, 2020;Published: February 07, 2020

ISSN: 2576-9162 Volume7 Issue3
Abstract
The immune response of Enterococcus faecium AL41 (EF AL41), Enterococcus faecium H31 (EF H31), and Lactobacillus fermentum AD1 (LF AD1) against Campylobacter jejuni CCM6191 (CJ) were investigated in vitro by flow cytometry and immunophenotyping of cultivated peripheral blood mononuclear cells (PBMC). It may be postulated that using of probiotic bacteria strains in single arrangement during 48h in vitro cultivation displayed different influence to evaluated T (CD3, CD4, CD8) and B (BU1, IgA, IgM) immune cells. E. faecium AL41 acted predominantly 24h pi with C. jejuni while L. fermentum AD1 effect was detected 48 h pi. Both probiotic strains also displayed distinct manner of T and B cells activation. E. faecium H3 showed the lowest effect to both, controls and C. jejuni.
Introduction
Chickens are a natural host for Campylobacter species and are often colonized by Campylobacter jejuni and Campylobacter coli [1]. Particularly broiler chickens are commonly regarded as a natural host for this zoonotic pathogen and infected birds carry a very high C. jejuni load in their gastrointestinal tract, especially the ceca. This results in contaminated carcasses during processing [2]. Meat contaminated with C. jejuni is an important source of foodborne gastroenteritis and poses a serious health burden in industrialized countries. C. jejuni isolates can have different potential of colonization and have been divided into three phenotypes. First phenotype fails to colonize 14-day-old chickens, second can colonize but are readily eliminated and are classified as transient, third show efficient and sustained colonization [3]. These three colonization phenotypes were found to be stable and independent of in vivo passages and the number of viable bacteria in the inoculum. Enhanced colonization capacity and increased virulence after in vivo passage through chicks has been shown [4]. The C. jejuni induced adaptive T-cell response in the intestine in ex vivo infected explants of human colon tissue. After reached subepithelial compartments, adhesion and invasion of intestinal epithelial cells follows. The direct contact of the invading bacteria with neutrophils, macrophages, and dendritic cells initiated Th1 lymphocytes and responses characterized by the release of IFN-γ, IL-1β, IL-12, and IL-6 [5]. An increased IL-12 and IL-23 level indicates the dendritic cell activation and maturation, which is subsequently driving the response of distinct Th17 cell subsets. Elevated numbers of Th17, Th1, and Th17/Th1 double-positive cells were well in line with increased concentrations of IL-22 and IL-17 which orchestrate the eradication of the pathogen by induction of innate responses and defensin production in the epithelium. A possible way to reduce Campylobacter contamination in poultry is to develop new actions at the primary production level. Therefore, it has become necessary to develop alternatives such as beneficial microorganisms (probiotics). The use of probiotics, which can help to improve the natural defence of animals against pathogenic bacteria, is an alternative and effective approach to antibiotic administration for livestock to reduce bacterial contamination. In vitro experiments showed that Enterococcus faecium, Pediococcus acidilactici, Lactobacillus salivarius, and Lactobacillus reuteri isolated from healthy chicken gut inhibited the growth of C. jejuni. In vivo administration of Enterococcus faecium 55 to chicks challenged with Salmonella Enteritidis 147 in combined group (EF+SE) ultimately resulted in increased number of bloods heterophiles, caecal IgA+ IEL, and decreased caecal expression of MUC compared to SE group on 7 days post infection. Detection of IL-15 and IL-17 mRNA cytokine level in caecum showed a tendency to their increase on 1-day post salmonella infection in combined EF+SE group [6]. The aim of our in vitro study was to determine by flow cytometry the stimulation of main T and B lymphocyte subpopulations in peripheral blood mononuclear cell culture after preventive administration of probiotic bacterial strains Enterococcus faecium AL41, Enterococcus faecium H31 and Lactobacillus fermentum AD1 and Campylobacter jejuni infection.
Material and Method
Blood was collected from the vena cutanea ulnaris to 1.5% EDTA from clinically healthy poultry reared under standard condition. Collected blood was diluted with PBS in a ratio of 1:2 and transferred to Leucoseps tube (Greiner bio-one, DE) containing histopaque-1077 (Sigma-Aldrich, UK) and centrifuged for 40min at 19000×g at 20 °C (Hettich Rotina 420R Centrifuge, DJB Labcare, UK). Mononuclear cells were collected from the gradient interface and washed 2 times for 5min at 16000 × g with PBS. Cell viability and number were determined by trypan blue exclusion. Isolated PBMC (Peripheral Blood Mononuclear Cells) were placed on 12-well cultivation plate (Orange Scientific, BE) at the concentration of 1x107 cells/mL and cultured overnight (39 °C, 5% CO2) in RPMI 1640 medium enriched with 10mM HEPES (Lonza, BE) and 10% FBS (Lonza, BE). Followed by 24 hours cultivation 200μl of particular probiotic bacterial strains (provided by Dr. Lauková, IAP SAS, Košice, Slovakia) were added to the individual PMBCs cultures. The concentrations of C. jejuni CCM6191 was 1x108 CFU/ml. Enterococcus faecium AL41, Enterococcus faecium H31 and Lactobacillus fermentum AD1 were added at the concentration of 1x109 CFU/ml. After the addition of probiotic bacteria PMBCs were cultured for 24 and 48 hours. The cultivation of bacterial strains, culture medium and growth conditions were performed as described previously [7,8].
Flow cytometry procedureMouse anti-chicken monoclonal antibodies CD3, CD4, CD8 (T-cells), BU1, IgA, IgM (B-cells) labelled with FITC (SouthernBiotech, USA) were used for immunophenotyping of lymphocytes by direct immunofluoerescent method. After cultivation the lymphocytes were washed twice with phosphate buffered saline (PBS). Fifty μL of cellular suspension (1.107 lymphocytes in PBS) and 2μl of specific or control MoAbs were mixed and incubated in dark at 22 °C for 15min. After being stained, the cells were washed once in 0.5mL PBS and resuspended in 0.2mL of PBS with 0.1% paraformaldehyde. Measurement and analysis of stained cells was performed by FACS system (Becton Dickinson, Germany) provided with a 15mV argon ion laser. The analysis examined a dot plot of the leukocytes obtained by the forward and side scattering of the physical character of the lymphocyte population. Gates were drawn around lymphocytes based on 90° and forward-angle light scatter. The fluorescence data were collected on at least 10,000 lymphocytes using the Becton Dickinson Cell Quest programme. The results are therefore expressed as the relative percentage of the lymphocyte subpopulation which was positive for a specific MoAb. Samples were measured in triplets before cultivation, 24h after cultivation, 24h and 48h post infection (pi) in following arrangement: controls, probiotic strain, campylobacter and also co-cultivation probiotic + campylobacter group. Statistical analysis was done using GraphPad Software and one-way analysis of variance (ANOVA) with the post hoc Tukey multiple comparison test. The differences between the mean values for the groups of chickens were considered significant when P<0.05.
Result
Subpopulations of lymphocytes in controls 24 hours after cultivation showed the increase of values in comparison with samples before cultivation (data not shown). Probiotic strain Enterococcus faecium AL41 24h pi (Figure 1) showed increase (***p<0.001) of all examined T-cell subpopulations (CD3+, CD4+, CD8+) and Bu1+ in combined EF AL41+CJ group in comparison with other groups. IgA expression was the highest in CJ group (***p<0.001 vs C and EF AL41+CJ, *p<0.05 vs EF AL41), and IgM+ subpopulation in EF AL41 group (*p<0.05 vs C, ***p<0.001 vs CJ and EF AL41+CJ). 48 h pi (Figure 2) was recorded the increase of examined subpopulations in experimental groups compared to control, except of IgA+ and IgM+ with the peak in C groups (***p<0.001). However, the highest values were found in CJ groups (***p<0.001), overstimulation persisted only in CD8+ of EF AL41+CJ group. Controls showed maximal IgA+ and IgM+ values while probiotic groups overexpressed CJ groups. Probiotic strain Enterococcus faecium H31 24 h pi (Figure 3) surprisingly overstimulated almost all determined lymphocyte subpopulations in control groups (***p<0.001) when compared to experimental groups, except BU+ cells showed maximum level (***p< 0.001) in EF H31 alone and combined probiotic group EF H31+CJ. Strain C. jejuni exceeded the expression of probiotic groups in CD4+, CD8+ and IgA+ subpopulations. 48 hours post infection (Figure 4) on the contrary upregulation showed experimental groups, mainly EF H31 (***p<0.001) in T-cell and IgA+ subpopulations compared to controls, CJ and EF H31+CJ. Infected CJ group reached the higher values in BU expression (***p<0.001) than control and probiotic groups. On the other hand, the values of IgM+ lymphocytes in EF H31 group were downregulated (***p<0.001 vs C, CJ, EF H31+CJ). Probiotic strain Lactobacillus fermentum AD1 24h pi (Figure 5) upgraded the subpopulations of experimental groups in comparison with controls except of slightly higher CD3 expression (**p<0.01 vs LF AD1, *p<0.05 vs CJ, ***p<0.001 vs LF AD1+CJ). Strain C. jejuni (CJ) overnumbered both probiotic groups only in CD4+ subpopulation (**p<0.01). Overstimulation of combine group LF AD1+CJ (***p<0.001) was observed in CD8+ T-cells, as well as BU+ and IgM+ B-cells, while IgA+ showed overexpression in alone probiotic LF AD1 group (***p<0.001) compared to others. 48 hours pi (Figure 6) the higher values of experimental groups persisted (***p<0.001) when compared to the controls. While CD3+, CD8+ and BU+ subpopulations showed overstimulation in LF AD1+CJ group (***p<0.001) followed by LF AD1, CD4+, IgA+ and IgM+ were overexpressed in CJ groups (***p<0.001).
Figure 1:
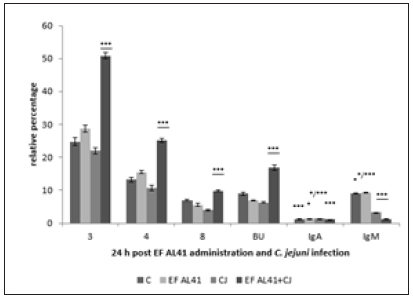
Figure 2:
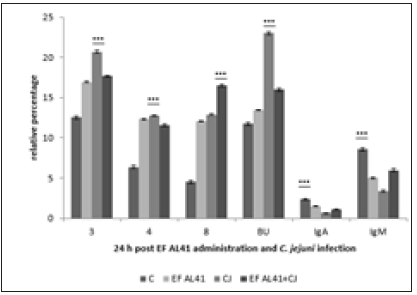
Figure 3:
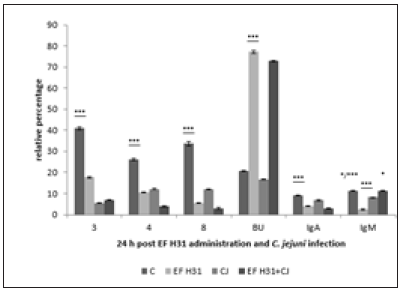
Figure 4:
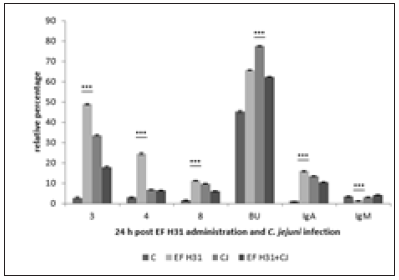
Figure 5:
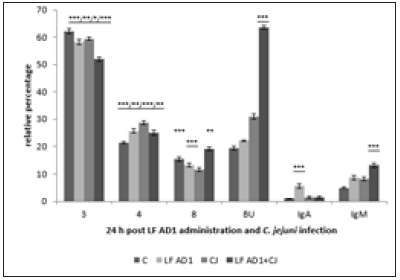
Figure 6:
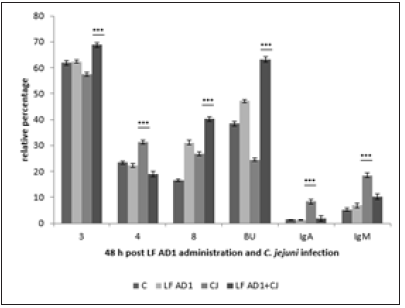
Discussion
Campylo bacteriosis is a collective description for infectious diseases caused by members of the bacterial genus Campylobacter. The only form of campylobacteriosis of major public health importance is Campylobacter enteritis due to C. jejuni and C. coli. The rate of Campylobacter infections worldwide has been increasing, with the number of cases often exceeding those of salmonellosis and shigellosis. This increase, as well as the expanding spectrum of diseases caused by the organisms, necessitates a clearer understanding of the epidemiology and control of campylobacteriosis [9]. Campylobacter jejuni are gram-negative, spiral, microaerophilic, motile bacteria and one of the most common enteric bacterial pathogens globally. The Campylobacter multidrug efflux pump (CME) plays an important role in multidrug resistance in C. jejuni, mediating resistance to heavy metals and a broad range of antibiotics and other antimicrobial agents [10]. It is also responsible for resistance to bile salts in the chicken intestinal tract and is therefore essential for successful intestinal colonization in chickens [11]. In mice, ferrets, nonhuman primates, and humans, immunity to sickness is suggested to be controlled by an immune mechanism distinct from that controlling immunity to infection. In ferrets C. jejuni CG8421 stimulated both mucosal and systemic antigen-specific IgA and IgG [12]. Hermans et al. [13] mentioned that if antibodies against C. jejuni are already present in the chick, the ability of C. jejuni to colonize is dramatically reduced, be it due to transfer of maternal antibodies to or through immunization of such birds. Our in vitro study showed the gradual increase of IgA+ lymfocytes in C. jejuni groups during cultivation that reached the peaks at 24 or 48h in all three experiments with different probiotic bacterial strains. In spite of probiotic strains overstimulation, we missed any prevalence of combined probiotic and C. jejuni groups. C. jejuni group also presented improvement of IgM, and BU1 subpopulations including into production of antibodies. LF AD1 strain upregulated BU1 subpopulation 24 and 48h post infection. Because genetically polymorphic cell surface antigen, BU-1, is expressed on B cells as well as on a subset of macrophages and no correlation with IgM was observed, we supposed also activation of macrophages. Overstimulation of macrophages proved Karaffová et al. [14] by demonstration that E. faecium AL41 can modulate the TLRs expression and modify the activation of MIF, IFN-γ, MD-2 and CD-14 molecules in the chicken caecum challenged with C. jejuni CCM 6191. A little information connected with C. jejuni we found in literature about cell mediated immune response, mainly subpopulation of lymphocytes in peripheral blood and immune organs in chickens. By some authors there is some similarity of immunity in C. jejuni to Salmonella infection when changes in numbers, distribution and proliferation of T cells and delayed type of hypersensitivity were observed [15].
We found stimulation of T cells in C. jejuni groups, mainly CD3, CD4, CD8, CD44, as well as higher expression of CD45 and MHC II antigens as the proof of specific cell immunity including in prevention against intracellular bacteria (unpublished data). Increase of MHC II antigens is important for their presentation of bacterial antigens to TH (CD4+) and TC (CD8+) lymphocytes. Activated TC then are able to kill infected macrophages tend to loss of protective atmosphere for bacteria that results to inhibition of infection or its dissemination.
Probiotics can be defined as live microbial feed supplements that beneficially affect the host animal by improving its intestinal health [16]. Different strains of the same species of probiotics can have unique biological activities, such as different sites of adhesion, specific immunological effects, and fermentation characteristics [17] Willis and Reid [18] showed that C. jejuni was present at a lower level in broiler chickens fed with a standard diet supplemented with a probiotic formulation containing L. acidophilus, L. casei, B. thermophilus, and E. faecium (108 cfu/g) with respect to the control. We have done in vitro co-culture of C. jejuni and three individual probiotic bacterial strains to determine and recognize their immune influence for using of the most effective strain in the future for in vivo arrangement experiments in chicks. The aim will be finding out the possible decrease of C. jejuni after oral administration these newest probiotic bacterial strains. Imunofenotyping of our studied probiotic bacterial strains E. faecium AL41, E. faecium EF H3, and Lactobacillus fermentum AD1 manifested different immunostimulatory effect when compared to C. jejuni and also controls. Many papers reported beneficial effect of probiotics, mainly in connection with reducing of C. jejuni shedding. Morishita et al. [19] reported that Avian PAC Soluble, which contains the bacteria L. acidophilus and S. faecium, was successful in reducing the shedding (70%) and jejunal colonization of C. jejuni in market-aged broilers. Morishita et al. [20] also published a 70% reduction in the frequency of C. jejuni in chicks with the use of a commercial probiotic containing L. acidophilus and E. faecium. In our previous in vivo experiment, we tested the effectivity of probiotic strain E. faecium AL41 (EF AL41) to S. Enteritidis PT4 (SE) infection in the peripheral blood of chickens. The probiotic groups (EF AL41 alone and combine EF AL41+SE) on 4 dpi showed tendency to improve expression of CD3, CD4, CD8, IgM, MHCII, as well as IgA subpopulation in EF group [21]. In vitro study with C. jejuni showed 24h pi the increase of all determined subpopulations -CD3, CD4, CD8, BU1, IgM, and also CD44, CD45 (data do not show) in probiotic groups excepting IgA that was higher in C. jejuni. Countertendency was observed 48 h pi when values C. jejuni group slightly outnumbered expression of determined subpopulations in probiotic groups except of IgA, IgM and CD44. Imunofenotyping of lymphocyte subpopulation of probiotic strain E. faecium H3 showed lower effectivity then EF AL41. Whereas the expression at 24h pi in probiotic groups was similar to detection of EF AL41 in CD3, Bu1, IgM, CD44 and CD45 and overnumbered values in C. jejuni, 48 pi higher expression showed only EF AL41 group in T lymphocytes (CD3, CD4, CD8), and IgA subpopulation. The testing of L. fermentum AD1 probiotic bacterial strain demonstrated better immunostimulation of LF AD1 and LF AD+CJ at 48h pi presented with increase of expression CD3, CD8, Bu1, CD44, CD45, and MHC I, whereas at 24h pi the improving was detected only in CD8, IgM and MHC I subpopulations. C. jejuni group on the contrary showed increase in CD4 and MHC II at 24h pi followed with rising of IgA and IgM at 48h. It may be supposed preference of humoral immunity in C. jejuni infection. We did the monitoring of mucosal immunity by determination of cecal intraepithelial (IEL) and lamina propria lymphocytes (LPL) in chickens administered with Lactobacillus fermentum CCM 7514 and Campylobacter coli challenge that indicated the improve of CD8+ IEL at 4 and 7 dpi in combined LBCB groups. Intraepithelial CD3+, CD4+, and IgA+ lymphocytes raised also in combined LBCB groups. Cecal LPL showed the highest values of IgA+ at 4 and 7 dpi together with IgM+ cells. Splenic CD4+ and IgA+ lymphocytes were increased at 7 dpi. The preliminary results suggested beneficial effect of L. fermentum CCM 7514 mainly by improving of humoral response [22]. In conclusion may be postulated that using of probiotic bacterial strains in single arrangement during 48h in vitro cultivation displayed different influence to immunocompetent cells. While E. faecium AL41 increased all T cells and BU1 populations 24 h pi, production of IgA and IgM was noticed 48h pi. E. faecium H3 showed minimum interference in comparison with controls that overexpressed the values of both EF H3 and EF H3+CJ probiotic groups except of Bu1, CD44 and CD45 at 24h, although at 48h alone EF H3 showed the highest expression of T cells and IgA subpopulations. On the contrary LF AD1 manifested 48h pi mainly activation across CD8 T lymphocytes with increase of BU1. Finally, E. faecium AL41 acts earlier then L. fermentum AD1 with distinct manner of activation of T and B cells.
Acknowledgement
This work was supported by Slovak Research and Developmental Agency APVV-15-0165, Grant Agency for Science of Slovak Republic VEGA 1/0112/1 and 1/0355/19.
References
- European Food Safety Authority (2010) The community summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in the European Union in 2008. EFSA J 8(1): 1496.
- Berrang ME, Buhr RJ, Cason JA, Diclens JA (2001) Broiler carcass contamination with Campylobacter from feces during defeathering, J Food Prot 64(12): 2063-2066.
- Hänel I, Bormann E, Müller J, Müller W, Pauly B, et al. (2009) Genomic and phenotypic changes of Campylobacter jejuni strains after passage of the chicken gut. Vet Microbiol 136(1-2): 121-129.
- Cawthraw SA, Wassenaar TM, Ayling R, Newell DG (1996) Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiol Infect 117(1): 213-215.
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, et al. (2005) Diversity of the human intestinal microbial flora. Science 308(5728): 1635-1638.
- Levkut M, Revajová V, Karaffová V, Lauková A, Herich R, et al. (2016) Evaluation of mucin and cytokines expression with intraepithelial lymphocytes determination in the caecum of broilers administered with Enterococcus faecium EF55 and challenge with Salmonella Enteritidis SE147. JVMAH 8(12): 214-222.
- Strompfová V, Marciňaková M, Gancarčíková S, Jonecova Z, Scirankova S, et al. (2005) New probiotic strain Lactobacillus fermentum AD1 and its effect in Japanese quail. Vet Med Czech 50(9): 415-420.
- Lauková A, Chrastinová L, Simonová MP (2012) Enterococcus faecium AL41: Its enterocín M and their beneficial use in rabbit’s husbandry. Probiotics Antimicro 4(4): 243-249.
- Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL (2002) Human campylobacteriosis in developing countries. Emerg Infect Dis 8(3): 237-243.
- Lin J, Michel LO, Zhang QJ (2002) CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother 46(7): 2124-2131.
- Lin J, Sahin O, Michel LO, Zhang QJ (2003) Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun 71(8): 4250-4259.
- Nemelka KW, Brown AW, Wallace SM, Jones E, Asher LV, et al. (2009) Immune response to and histopathology of Campylobacter jejuni infection in ferrets (Mustela putorius furo). Comp Med 59(4): 363-371.
- Hermans D, Deun KV, Martel A, Van Immerseel F, Messens W, et al. (2011) Colonization factors of Campylobacter jejuni in the chicken gut. Review Vet Res 42: 82.
- Karaffová V, Marcinková E, Bobíková K, Herich R, Revajová V, et al. (2017) TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and sIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiol 62(2): 89-97.
- Berndt A, Wilhelm A, Jugert CH, Pieper J, Sachse K, et al. (2007) Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect Immun 75(12): 5993-6007.
- Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66(5): 365-378.
- Isolauri E, Salminen S, Ouwehand AC (2004) Microbial gut interactions in health and disease. Probiotics. Best Pract Res Clin Gastroenterol 18(2): 299-313.
- Willis WL, Reid L (2008) Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni Poult Sci 87(4): 606-611.
- Morishita TY, Aye PP, Harr BS, Cobb CHW, Clifford JR (2000) Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis 41(4): 850-855.
- Morishita TY, Aye PP, Harr BS, Cobb CW, Clifford JR (1997) Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuni in broilers. Avian Dis 41(4): 850-855.
- Revajová V, Husáková E, Stašová D, Spišáková V (2014) Flow cytometry immunophenotyping of in vitro cultivated lymphocytes after Campylobacter jejuni infection with some probiotic bacteria strains. pp. 55-60.
- Revajová V, Gancarčíková S, Karaffová V (2017) Study of immune response in blood, spleen and cecum to Lactobacillus fermentum CCM 7514 in chickens infected with Campylobacter spp. pp. 21:139.
© 2020 Viera Revajová. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






