- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Ursolic Acid: Efficacy and Stability as a Preservative Against Mold and Yeast in Poultry Feed
Lotte Geerlofs1,2, Zhiyong He1,2*, Sa Xiao1,2, Vivan Liu1,2 and Zhi Cheng Xiao1,2*
1Department of Anatomy and Developmental Biology, Australia
2IRiccorgpharm Health Pty Ltd, Australia
*Corresponding author: Zhiyong He, Department of Anatomy and Developmental Biology, Australia; Zhi Cheng Xiao, Department of Anatomy and Developmental Biology, Australia
Submission: December 2019;Published: December 16, 2019

ISSN: 2576-9162 Volume7 Issue3
Abstract
Consumers have been demanding safer alternatives for chemical preservatives. There are natural compounds that could serve as preservatives in the future. The objective of this study was to investigate the efficacy of ursolic acid, either alone or in combination with berberine, against mold and yeast in poultry feed. First, the stability of ursolic acid was tested using HPLC to determine the concentration of ursolic acid in the feed. Furthermore, ursolic acid was mixed into the feed at a 0.05g/kg concentration or in combination with berberine at a concentration of 0.03g/kg berberine and 0.05g/kg ursolic acid. A solution containing mold and yeast was added to each bag of feed. The mold and yeast concentration were determined at different time points. Addition of 0.05g/kg of ursolic acid to the feed significantly inhibited the growth of mold but not the growth of yeast. When combined with 0.03g/kg berberine, 0.05g/kg ursolic acid significantly inhibited the growth of both mold and yeast.
keywords Ursolic acid; Natural preservative; Antimicrobial; Stability; Efficacy
Introduction
Food preservation has changed over the years. Whereas physical methods used to be the standard, the food industry turned to chemical compounds to preserve and/or enhance flavour of foods beginning in the early 1800s [1]. There are two major government bodies that regulate the use of food additives and ingredients: the Food and Drug Administration (FDA) in the United States and the European Food Safety Authority (EFSA) in Europe. FDA regulates feed ingredients as either approved food additives, prior sanctioned substances, “Generally Recognized as Safe (GRAS) ingredients or substances subject to Official Definition in the AAFCO Official Publication. EFSA divides all feed additives in four groups regarding their origin and manufacture: natural additives, similar to natural additives (but produced synthetically), chemically modified natural additives and artificial additives Carocho et al. [2].
Chemical compounds /artificial additives are most often used as preservatives but are known to cause harmful effects Di Sotto et al. [3], Antunes et al. [1], Mellado et al. [4]. Certain natural compounds have been established as GRAS under the conditions of use in feeds and preferred over unsafe chemical compound by consumers. This has resulted in a higher demand for natural alternatives and an increased amount of studies performed to investigate environmentally friendly and natural food additives as preservatives Lai et al. [5]. An example is the investigation of clove in preserving the colour of raw pork Shan et al. [6].
There are a number of requirements for a natural preservative: apart from safety, it must have stability during food processing as well as antimicrobial efficacy Lee & Paik [7]. In turn, there are several factors that have an effect on the efficacy of a preservative. The best preservation is achieved when micro-organisms are inhibited, all micro-organisms and their concentration are known, optimum storage conditions and an optimum pH Davidson et al. [8].
Ursolic acid (UA) is a pentacyclic triterpenoid compound and can be found in various types of fruit Jezus et al. [9]. UA has been challenged against common food pathogens and showed a moderate antimicrobial effect Subramaniam et al. [10], but it has not been studied extensively as a preservative.
The objective of this study was to investigate the efficacy of UA as a preservative in poultry feed. The aims were to test the stability of UA in poultry feed for 14 days followed by a challenge study with or without berberine against mold and yeast strains over 56 days.
Material and Method
The feed was manufactured at SPR feed mill and the feed samples were provided by Southern Poultry Research, Inc. The pelleted feed was produced from mash using steam conditioning (5” diameter x 36” length) and a pellet mill (Model CL5 California Pellet Mill Co., Crawfordsville, IN) equipped with a 5/32”x 7/8” die for broiler diets. Between each of the treatments, a 20lb flush was run. There was a constant rate of 2Ibs per minute added to the pellet mill from the hopper that contained the mash feed. 185F was the target conditioning temperature and was achieved by increasing steam addition. Cooling trays with pellets exited the pellet die and were cooled at room temperature for 10 minutes in a counter-flow cooler.
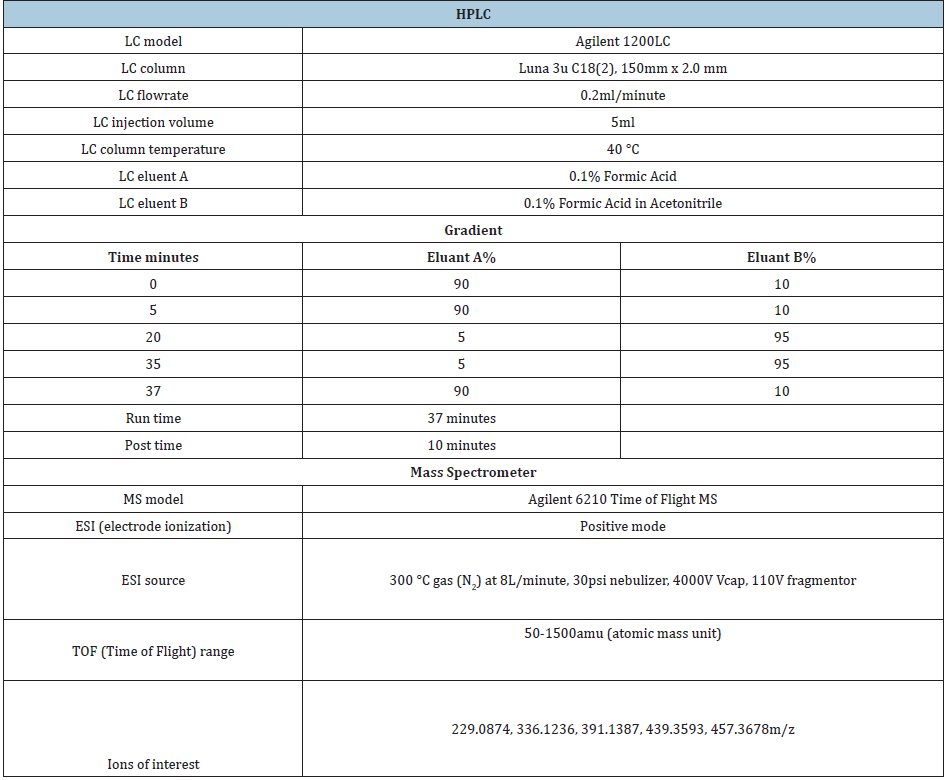
StabilityAspen research provided the standards with certificates of analysis. The samples used are listed in Table 1. UA was prepared in methanol in triplet for the individual analytical standards. Those three samples were combined and that combined standard was used to make serial dilutions in methanol. Each sample was analysed in duplicate. 10mL of methanol was used to extract 1g of sample. Before weighing the pellet samples, they were mashed into a powder like consistency using a mortar and pestle. Using a wrist-action shaker, the mixture of methanol and the samples were shaken for 30 minutes. After 10-minute waiting period, the methanol was decanted off into a separate vial.
Table 1: Official name and ATCC number of the used mold and yeast strains for the inoculation cocktail.

UA acid 0.05g/kg and the combination group of 0.03g/kg berberine and 0.05g/kg UA were diluted in 10:1 in methanol. UA 0.15g/kg was diluted 100:1 methanol. All samples were filtered through a 0.2m PTFE syringe prior to analysis. All standards and samples were injected to a LCMS using conditions in Table 2.
Table 2: Samples of UA and berberine used to determine the stability of the concentration in the feed.
Efficacy
The protocols provided by Deibel labs were followed to grow and harvest the yeast and mold cultures. Several mold and yeast cultures (listed in Table 3) were combined to create an inoculation cocktail. To form a spore suspension for each individual culture, a culture was filtered through a sterile cheese cloth. To verify the spore culture, lactophenol aniline was added to a drop of culture and a coverslip was placed over the mixture. Then, the slide was analyzed under a microscope to identify hyphae presence. The culture was filtered again if there was a significant amount of hyphae present. After the verification of the spore culture, the mix was diluted to the desired inoculum level.
Table 3: The HPLC settings used to determine the stability of the concentration in the feed.
Each sample that was used for the study consisted of 50 grams of feed in a sterile whirl-pak bag. Depending on if the sample was part of the control or treatment group, the mold or yeast cocktail was added. 0.25ml of the cocktail was added drop wise to different locations within the bags containing the test groups samples. The bags were massaged to mix the feed and the mold/yeast cocktail. To ensure a closed environment, the bags were rolled closed. The target start concentration in each test bag was 102-103 CFU/g of the mold or yeast cocktail.
At day 0, all samples were plated. On day 7,14,21,28,42 and 56 a sample was taken from each bag, and 450 ml of Butterfields Phosphate Buffered Diluent (BPB) was added to each sample. For 1-2 minutes, BPB and the sample were homogenized and were plated immediately after. YM agar plates were used to plate the yeast samples and Potato Dextrose Agar plates with a chlortetracycline additive was used to plate the mold samples. After being incubated at 25 °C for five days, the plates were enumerated. Samples of the negative control were taken at the beginning and at the end of the study. The samples were plated and incubated the same way as the test samples to determine background flora samples.
The samples were stored at ambient temperature in a high humidity chamber. Inside the chamber, an open pan with water was placed to ensure the high ambient humidity. On the sample days, the samples were taken in triplicate. In addition to that, the samples were observed for visible mold or yeast growth.
StatisticsTo compare the test and control groups at every time point, an unpaired T-test was performed. The results were considered significant if p <0.05. The graphs were generated in Graphpad Prism version 8 (Graphpad software Inc., USA).
Result
The concentration measured in the feed to determine the stability are presented in Table 4. If the concentration of UA was below 0.005g/kg, it could not be measured. Therefore, if it was below 0.005, it was reported as not detected (N.D). The results showed that UA is stable when stored at room temperature over the two-week storage time.
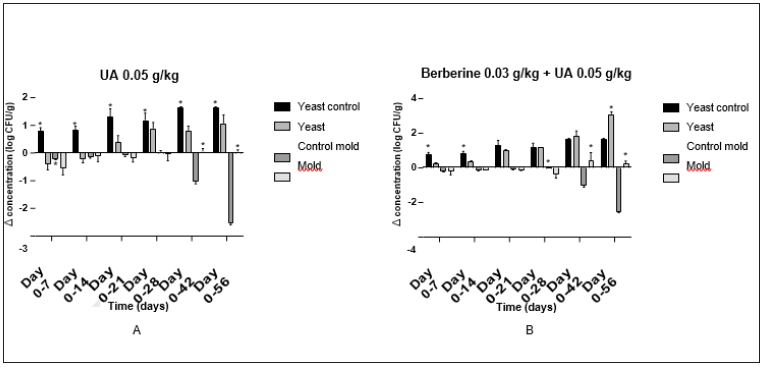
EfficacyThe pellet samples were tested and the graphs are presented in Figure 1. The 0.05g/kg group (5A) showed that the control group was significantly reducing the yeast concentration at all time points throughout the experiment. However, the test group was performing better in reducing the mold concentration at day 42 (p<0.001) and 56 (p<0.001). The combination group of UA and berberine (5B) showed that for the first half of the experiment, the control group was performing better at significantly reducing the mold or yeast concentration than the test group. After day 28, the test group was performing better at reducing the mold concentration at both day 42 (p<0.001) and 56 (p<0.001). At day 56, the test group was significantly performing better in reducing the yeast concentration in the feed (p<0.001).
Table 4: Measured concentrations of UA and berberine in the feed.
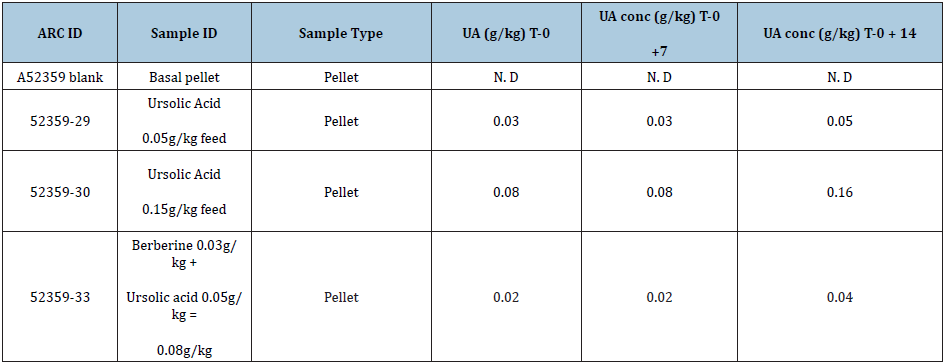
N.D = not detectable.
Figure 1: A: The effect of 0.05g/kg UA on mold and yeast growth. The control group was performing better at reducing the yeast concentration. The test group was significantly performing better at reducing the mold concentration at day 42 and 56. B: The effect of berberine and UA on the growth of mold and yeast. The control group was performing better for the first half of the experiment. For the second half of the experiment, the test group was preforming better than the control group at significantly reducing the mold and yeast concentration. All values are mean ± SD. * indicates a significant difference.
Discussion
Currently, over 2500 additives are added to foods as additives. Many of those additives have been banned over the years on a global or continent scale. In the last decade, a lot of studies have proven the harmful effects of some of the chemical preservatives used in food Etemadi et al. [11], Varraso & Camargo [12]. Therefore, there is an increased interest in natural compounds that can be used as preservatives, such as plant extracts, purified secondary metabolites or essential oils Gassara et al. [13], Carocho et al. [2]. Nonetheless, there are still a number of requirements that a natural preservative must satisfy: apart from safety, it must have stability during food processing as well as antimicrobial efficacy.
The experiment conducted to test the stability of the concentration of UA in the feed showed that UA is stable for at least 14 days under the experimental conditions employed. This study showed that 0.05g/kg of UA incorporated in poultry feed, regulates the mold concentration over 56 days. The mold concentration did not increase whereas the mold concentration of the control group did increase. In addition to that, the combination group of UA 0.05g/ kg and berberine 0.03g/kg also showed preservative properties since the yeast concentration and mold concentration significantly decreased during the second half of the experiment. This study has showed that UA has a significant effect on the concentration of mold and poultry feed and is stable. Preservative effects were also observed with a combination of UA and berberine, which was also found to be stable [14].
Acknowledgement
This research was conducted with the help of Deibel Laboratories Gainesville. Conflict of interest No declaration of competing and conflicting interests. This research was supported by iRiccorgpharm Health Pty Ltd in its initiative to reduce antibiotic usage in livestock. They had no influence on the results and statistical analysis.
Authors’ Contributions
Zhiyong He designed the experiment. The study was conducted upon request from Sa Xiao and Zhi-Cheng Xiao. Lotte Geerlofs wrote the manuscript on consultation with Zhiyong He and made revisions when necessary. Lotte Geerlofs, Vivan and Sa Xiao analyzed the data. All authors have read and approved the manuscript.
References
- Antunes SC, Nunes B, Rodrigues S, Nunes R, Fernandes J, et al. (2016) Effect of chronic exposure to benzalkonium chloride in Oncorhynchus mykiss: cholinergic neurotoxicity, oxidative stress, peroxidative damage and genotoxicity. Environmental Toxicology and Pharmacology 45: 115-122.
- Carocho M, Filomena Barreiro MF, Morales P, Ferreira ICFR (2014) Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Comprehensive Reviews in Food Science and Food Safety 13(4): 377-399.
- Di Sotto A, Maffei F, Hrelia P, Di Giacomo S, Pagano E, Borrelli F, Mazzanti G (2014) Genotoxicity assessment of some cosmetic and food Regulatory toxicology and pharmacology 68(1): 16-22.
- Mellado-García P, Maisanaba S, Puerto M, Prieto AI, Marcos R, et al. (2017) In vitro toxicological assessment of an organosulfur compound from Allium extract: cytotoxicity mutagenicity and genotoxicity studies. Food and chemical toxicology.
- Lai L, Chiu J, Chiou RY (2017) Fresh preservation of alfalfa sprouts and mushroom slicec by soaking with thymol and resveratrol solutions. Food Science & Nutrition 5(3): 776-783.
- Shan B, Cai Y, Brooks JD, Corke H (2009) Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. Journal of the Science of Food and Agriculture 89(11): 1879-1885.
- Shan B, Cai Y, Brooks JD, Corke H (2009) Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. Journal of the Science of Food and Agriculture 89(11): 1879-1885.
- Davidson PM, Taylor TM, Schmid, SE (2013) Chemical preservatives and natural compounds. Food Microbiology; Fundamentals and Frontiers.
- Jezus JA, Lago JHG, Laurenti MD, Yamamoto ES, Passero LFD (2015) Antimicrobial Activity of Oleonolic and Ursolic Acid: an Update. Evidence Based Complentary and Alternative Medicine 2015: 14.
- Subramaniam S, Rajendran N, Muralidharad SB, Subramaniam G, Raju R, Sivasubramanian A (2015) Dual role of select plant-based nutraceuticals as antimicrobial agents to mitigate food borne pathogens as food preservatives. Royal Society of Chemistry 94.
- Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue-Choi M, et al. (2017) Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population-based cohort study. BMJ (Clinical research ed.) 357: j1957.
- Varasso R, Camargo CA (2014) Processed meat consumption and lung health: more evidence for harm. The European respiratory
- Gassara F, Kouassi AP, Brar SK, Belkacemi K (2016) Green Alternatives to Nitrates and Nitrites in Meat-based Products-A Review. Critical reviews in food science and nutrition 56(13): 2133-2148.
- Sullivan GA, Jackson AL, Zhou GH, Sebranek JG (2011) Use of natural antimicrobials to improve the control of Listeria monocytogenes in a cured cooked meat model system. Meat Science 88(3): 503-511.
© 2019 Zhiyong He. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






