- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Piceid as a Preservative; Efficacy against Mold and Yeast in Poultry Feed
Lotte Geerlofs1,2, Zhiyong He1,2*, Sa Xiao1,2, Vivan Liu1,2 and Zhi Cheng Xiao1,2*
1Department of Anatomy and Developmental Biology, Australia
2Riccorgpharm Health Pty Ltd, Australia
*Corresponding author: Zhiyong He, Department of Anatomy and Developmental Biology, Australia; Zhi Cheng Xiao, Department of Anatomy and Developmental Biology, Australia
Submission: November 26, 2019;Published: December 04, 2019

ISSN: 2576-9162 Volume7 Issue2
Abstract
There has been an increased interest in investigating safer alternatives for chemical preservatives. It has been known that chemical preservatives have negative effects on the health of the consumer. The objective of this study was to investigate the efficacy of piceid, either alone or in combination with berberine, against mold and yeast in poultry feed. Piceid was mixed into the feed at a concentration of 0.5g/kg or in combination with berberine at a concentration of 0.5g/kg picied and 0.03g/kg berberine. A cocktail of yeast and mold was added to each bag. The concentration of mold and yeast was determined at different time points. In addition to that, the stability of the concentration of piceid was determined using HPLC. The 0.5g/kg concentration and the combination group with berberine showed significant preservative properties against mold and yeast. Furthermore, the concentration of piceid was stable in the feed for tested period.
keywords Piceid; Natural preservative; Antimicrobial; Stability; Efficacy
Introduction
The preservation of food is defined as applying different techniques to prevent spoilage and deterioration of food to extend the shelf life. In addition to that, is must be free of pathogenic micro-organisms Sancho [1]. Natural ways of preserving food without any additives can be achieved by boiling, dehydrating, smoking, pasteurizing and freezing food Sharma [2]. However, using preservatives as additives to extend the shelf life of a product is much more common. Chemical preservatives are most often used but there are some known harmful effects that are caused by certain kinds of preservatives. Nitrites and nitrate are being linked to stomach cancer (but with conflicting reports), benzoates may cause allergies and sorbates/ sorbic acids have been associated with contact dermatitis [3-7]. Therefore, a natural preservative without harmful effects is preferable.
Piceid, or polydatin, was originally found and isolated from the root and rhizome of Polygonum cuspidatum and is found in peanuts, grapes and chocolate products [8]. There are several derivates of piceid; trans-polydatin, trans-reservatrol, cis-polydatin and cis-reservatol. A study performed by Mikulski & Molski [9] showed that the trans-isomers have an increased bioactivity in comparison with the cis isomers Mikulski & Molski [9]. Berberine has been described previously Geerlofs et al. [10]. The aim of this study was to test the stability of piceid in poultry feed for 14 days followed by a challenge study of picied either alone or in combination with berberine against mold and yeast strains to investigate the efficacy of piceid as a preservative in poultry feed.
Materials and Method
The feed was manufactured at SPR feed mill and the feed samples were provided by Southern Poultry Research, Inc. Each batch of feed for this study was mixed and bagged separately. Pelleted feed was produced from mash via steam conditioning (5” diameter x 36” length) and subsequently use of a pellet mill (Model CL5 California Pellet Mill Co., Crawfordsville, IN) equipped with a 5/32”x 7/8” die for broiler diets. Mash feed was placed in the hopper of the pellet mill and the feeder was set at a constant rate to achieve approximately 2lbs. per minute. The target conditioning temperature of a 185F was achieved by adjusting (increasing) steam addition and conditioning time was approximately 30 seconds. Pellets were collected in cooling trays as they exited the pellet die. Pellets were cooled with ambient air for approximately 10 minutes in counter-flow cooler. A 20lb flush was run between each of the treatments.
StabilityThe samples with the certificates of analysis were provided by Aspen and the samples are listed in Table 1. Each sample of piceid was dissolved in 80:20 methanol: water solution. Three samples of the individual stock standards were prepared and combined to create a combined standard. That standard was used to prepare the dilutions in methanol for the experiment. The samples used were originally in pellet form but were crushed with a mortar and pestle. To extract 1g of sample, 10mL of methanol was used. The samples were shaken for at least half an hour and allowed to settle for 10 minutes. Afterwards, the methanol was removed and transferred into a separate vial. Piceid (0.5g/kg) and the combination group of berberine (0.03g/kg) and piceid (0.5g/kg) were diluted in 10:1 in methanol. All samples were filtered through a 0.2mm PTFE syringe prior to analysis. All standards and samples were injected to a LCMS using conditions in Table 2. Samples from each concentration were taken on day 0,7 and 14.
Table 1: Samples of berberine and piceid used to determine the stability of the concentration in the feed.
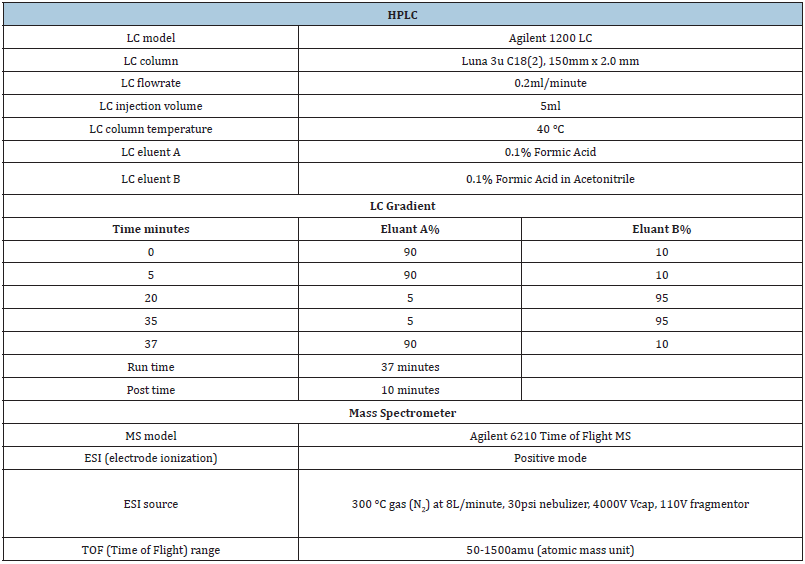
Table 2: The HPLC settings used to determine the stability of the concentration in the feed.
Efficacy
The mold and yeast organisms were cultured and accumulated according to the protocols provided by Deibel Labs and presented in Table 3. To form a spore suspension, the accumulated mold cultures were filtrated through a sterile cheese cloth. The separately harvested suspensions were combined to create an inoculation cocktail. 50μl of the inoculation cocktail was put onto a slide and lactophenol aniline was added to the slide. The combination of lactophenol aniline and the cocktail was then mixed, and the slide was sealed with a cover slip. The slide was analyzed under a microscope to determine the number of hyphae present. A significant number of hyphae could indicate that a spore culture wasn’t achieved. The cocktail was filtrated for a second time if the amounts of hyphae was too high. After confirming the spore culture, the solution was diluted to attain the target concentration. Each sample consisted out of a sterile whirl-pak bag containing 50 grams of feed. The control group samples were bags with feed that did not contain piceid and the test samples were bags with feed that did contain piceid. The target concentration per bag was 102-103CFU/g. 0.25ml of the mold or yeast cocktail was added to each sample bag and was spread throughout the entire bag. The bags were massaged to ensure the cocktail covered all the product. The bags were closed by using the rolling technique. The bags were stored at room temperature in a closed chamber that had an open pan containing water to ensure high humidity.
Table 3: Official name and ATCC number of the used mold and yeast strains for the inoculation cocktail.
Samples from the test bags were taken on day 0,7,14,21 and 28 and plated. Samples from the control bags were taken on day 0 and on day 28 to establish the presence of background flora. All samples were taken in triplicate. On day 7,14,21 and 28, 450mL of Butterfields Phosphate Buffered Diluent (BPB) was added to the sample. After 1-2 minutes the samples were plated. Those plates were then spread plated onto new plate. The samples that were inoculated with mold were plated onto a Potato Dextrose Agar plate with a chlortetracycline additive. The samples that were inoculated with yeast were plated onto a YM agar plate. The test and control plates were enumerated after incubation at 25 ºC for 5 days.
StatisticsTo compare the test and control groups at every time point, an unpaired T-test was performed. The results were considered significant if p <0.05. The graphs were generated in GraphPad Prism version 8 (GraphPad software Inc., USA).
Results
Table 4: Concentration of piceid in feed and in the combination feed with berberine.
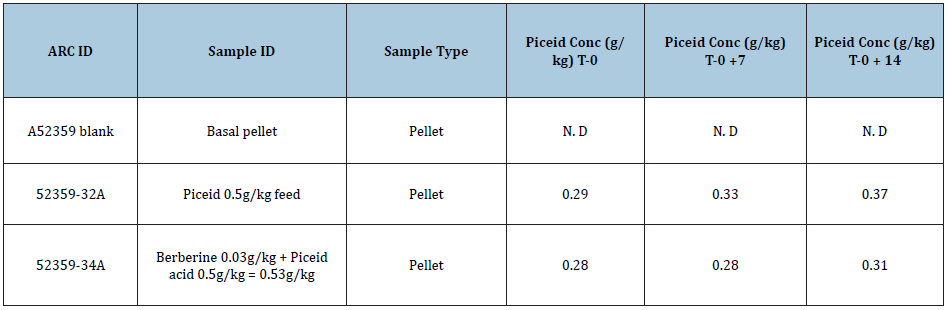
The results of the testing are summarized in Table 4. The method used can detect 0.005g/kg of additive. If the response was below that, the compound was reported as not detected (N.D). The results showed that piceid is stable when stored at room temperature over the two-week storage time.
EfficacyThe pellet samples were tested, and the graphs are presented in Figure 1. The 0.5g/kg group (5A) showed that the test group was performing significantly better than control group in reducing the yeast concentration at day 14 (p<0.001) and day 28 (p= 0.037). Furthermore, the test group was performing better in reducing the mold concentration at day 14 (p<0.001) and 21(p<0.001). The combination group of berberine and piceid (5B) showed that the control group was performing significantly better than the test group at day 7 (p<0.001) at reducing the yeast concentration. The test group performed better at reducing the yeast concentration at the end of the experiment. From day 14 until the end of the experiment, the test group had more effect against the mold than the control group.
Figure 1:
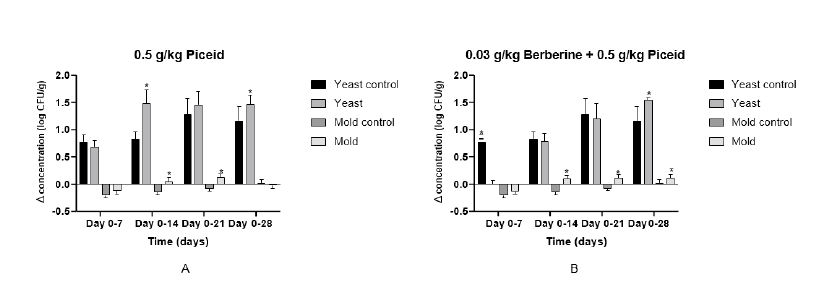
Discussion
There are two classes of preservatives: class I and class II. Class I preservatives are from a natural source and class II preservatives are chemical or synthetic preservatives. An item intended for consumption can have only one class II preservative otherwise there is a chance it might affect the overall health of the consumer Anand & Sati [11]. The harmful effects of chemical or synthetic preservatives in food and medicine have been well documented. Researchers have linked some preservatives to type 2 diabetes Tirosch et al. [12], prevalence of asthmatic attacks Nagel et al. [13], increased anxiety and motor impairment Noorafshan et al. [14]. All the industries that use preservatives in their products have been responding to the customers resistance and have been investigating the possibility replacing the harmful compounds with natural compounds [11]. Piceid could be one of the replacements compounds the results of this study showed that piceid does significantly reduces mold and yeas concentration at a concentration of 0.5g/kg incorporated into the feed. Furthermore, the combination group of berberine and piceid also decreased the concentrations significantly. In addition to that, the stability experiment showed that the concentration of piceid was stable for at least 14 days under the experimental conditions employed. This study has showed that piceid has a significant effect on the concentration of mold and yeast when incorporated in poultry feed and is stable. Good preservative effects were observed at a piceid concentration of around 0.5g/kg. Preservative effects were also observed with a berberine piceid combination, which was also found to be stable [15-19].
Acknowledgement
This research was conducted with the help of Deibel Laboratories Gainesville. Conflict of interest. No declaration of competing and conflicting interests. This research was supported by iRiccorgpharm Health Pty Ltd in its initiative to reduce antibiotic usage in livestock. They had no influence on the results and statistical analysis.
Authors’ Contributions
Zhiyong He designed the experiment. The study was conducted upon request from Sa Xiao and Zhi-Cheng Xiao. Lotte Geerlofs wrote the manuscript on consultation with Zhiyong He and made revisions when necessary. Lotte Geerlofs, Vivan Liu and Sa Xiao analyzed the data. All authors have read and approved the manuscript.
References
- Ott WH, Kuna S, Porter CC, Cuckler AC, Fogg DE (1956) Biological studies on nicarbazin, a new anticoccidial agent. Poult Sci 35(6): 1355-1367.
- Dougald LR, Fitz CS (2013) Protozoal infections. In Diseases of Poultry In: David ES (Ed.), (13th edn) Agricultural Research Service, USA pp. 1147-1201.
- Yoder CA, Graham JK, Miller LA (2006) Molecular effects of nicarbazin on avian reproduction. Poult Sci 85(7): 1285-1293.
- Vázquez G, Pérez PR (2000) Estimated gasometric values for the main populations and sites at higher altitude in Mexico. Rev Inst Nal Enf Resp 13(1): 6-13.
- Aviagen (2010) Reproduction-ROSS TECH investigation of incubation practices: Fertility evaluation. Midlothian, UK, p.48.
- Hamburger V, Hamilton H (1951) A series of normal stages in the development of the chick embryo. J Morphol 88(1): 49-92.
- Scott TA, Mackenzie CJ (1993) Incidence and classification of early embryonic mortality in broiler breeder chickens. Br Poult Sci 34(3): 459-470.
- Bellairs R, Osmond M (2014) Atlas of chick development. (3rd edn), United Kingdom, p. 692.
- (2011) SAS Institute Inc. Base SAS®3 Procedures guide. USA.
- Avery ML, Keacher KL, Tillman EA (2008) Nicarbazin bait reduces reproduction by pigeons (Columba livia). Wildlife Res 35: 80-85.
- Hughes BL, Jones JE, Toler JE, Solis J, Castaldo DJ (1991) Effects of exposing broiler breeders to nicarbazin contaminated feed. Poult Sci 70(3): 476-482.
- Jones JE, Solis J, Hughes BL, Castaldo DJ, Toler JE (1990) Reproduction responses of broiler-breeders to anticoccidial agents. Poult Sci 69(1): 27-36.
- Chapman DH (1994) A review of the biological activity of the anticoccidial drug nicarbazin and its application for the control of coccidiosis in poultry. Poult Sci Rev 5(4): 231-243.
- Flota BC, Ramírez MM, Dorantes JJ, José GG, Bautista O, et al. (2016) Description and diversity of family plots in rural areas of Campeche in Mé Agroproductividad 9: 38-43.
- Mata EA (2018) Phenotypic characterization and productive behavior of backyard chickens in the state of Campeche. Mé
© 2019 Zhiyong He. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






