- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Effect of Nicarbazin on Incubation Variables of Eggs from Creole Hens of Mexico
Martínez A1, Cuca García JM1, Matus Aragón MA1, Zárate Contreras D1, Sosa Montes E2, Osio Orihuela L2, Reyes Bello JG2 and González Cerón F2*
1Postgraduate in Livestock, México
2Department of Zootechnics, México
*Corresponding author: González Cerón F, Department of Zootechnics, México
Submission: November 04, 2019;Published: November 12, 2019

ISSN: 2576-9162 Volume7 Issue2
Introduction
Nicarbazin (NCZ) is an equimolar complex of 4,4’-dinitrocarbanilide and 2-hydroxy-4,6- dimethylpyrimidine Ott et al. [1]. It is an anticoccidial agent used in the poultry industry. Its main function is to prevent multiplication and proliferation of parasites of Eimeria genus in the intestinal tract, avoiding tissue damage, decreased feed consumption, reduced nutrient absorption, dehydration, blood loss, skin depigmentation and susceptibility to other pathogens in birds McDougald & Fitz-Coy [2]. Nevertheless, its application has not been entirely beneficial. Ott et al. [1] reported that feeding laying hens with 100 ppm or more of NCZ caused depigmentation of the brown shell and reduced hatchability in approximately 60%. Although the mode of action of NCZ for contraceptive activity is unknown, micrographs of vitelline membranes in mallards showed severe degenerative changes and preliminary in vitro studies indicated that it may increase intracellular calcium levels and early degradation of very low-density lipoproteins that comprise egg yolk Yoder et al. [3]. However, the effect of NCZ has not been studied in Creole hens of Mexico. Therefore, the objective of this investigation was to evaluate the effect of NCZ on incubation variables of eggs from that genotype of birds.
Materials and Method
This study was conducted at the Poultry Experimental Farm of the Chapingo Autonomous University at Texcoco, State of Mexico, Mexico. Located at an altitude of 2,247 m Vázquez G & Pérez P [4]. A total of 1922 eggs were collected from a population of 100 Creole hens of 80 weeks of age. Two treatments were evaluated: T2 (Control), 0.00% nicarbazin, and T1, 0.05% of nicarbazin in feed. Nine hundred and sixty-one eggs from the same population were used per treatment. The collection of eggs for T1 lasted 14 days, followed by a cleaning period of 21 days (feed without nicarbazin) and subsequently collecting eggs for T2. Storage was at an average temperature of 68 °F. The fertilization of the eggs was carried out by artificial insemination. Artificial incubation with a constant temperature of 100 °F and 60% Relative Humidity (RH) was used until day 18, then live embryos were passed to the hatcher (100 °F and RH: 80%). At 12 and 18 days of incubation eggs were candled to identify clear eggs and eggs with dead embryos, then the embryo diagnostic was carried out. Finally, at 21 days of incubation, nonhatched eggs were separated to conclude the embryo diagnostic. Early dead embryo with no blood (ED), 1-3 days of incubation; Early dead embryo with blood (EDB), 4-12 days of incubation Aviagen [5], Intermediate embryonic death (IED), 13-17 days of incubation; late embryonic death (LED) 18-21 days of incubation; Infertile (INFERT); Contaminated (CONT) and Hatched eggs (HATCH) Hamburger & Hamilton [6], Scott & Mackenzie [7], Bellairs & Osmond [8] were the categories considered in the embryo diagnostic.
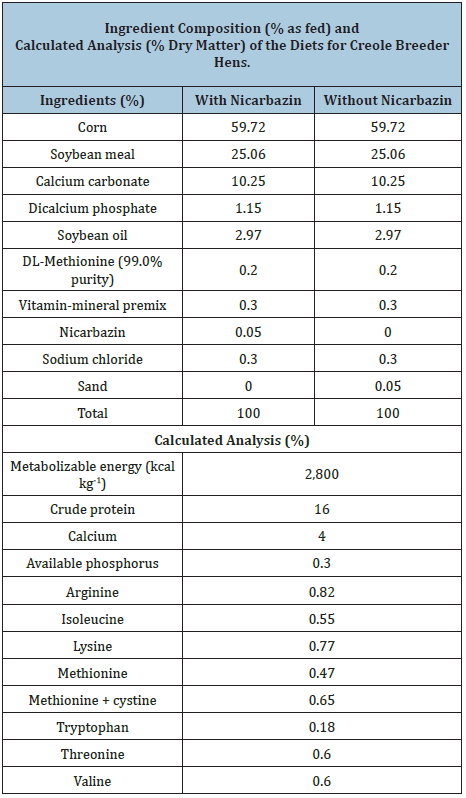
The feeding program consisted of a single phase with two diets, one containing 0.05% nicarbazin and one without nicarbazin, both diets containing 16% crude protein; 2,800 kcal ME kg-1 and 4% Ca (Table 1). They were provided with a program of 16 light hours. Water was supplied for free access and feed was restricted to 110 g bird-1 day-1.
1Provided per kg of diet: vitamin A, 12,000IU; vitamin D3, 1,000IU; vitamin E, 60IU; vitamin K, 5.0mg; vitamin B2, 8.0mg; vitamin B12, 0.030mg; pantothenic acid, 15mg; niacin, 50mg; folic acid, 1.5mg; choline, 300mg; biotin, 0.150mg; thiamine, 3.0mg. Fe, 50.0mg; Zn, 110mg; Mn, 100mg; Cu, 12.0mg; Se, 0.3mg; I, 1.0mg. 2Sand was used as an inert filler.
Table 1:
Statistical Analysis
Frequency data of categories from embryo diagnostic were compared by the chi-square test for independence with the Proc Freq contingency tests with α=0.05, SAS Institute Inc [9].
Results
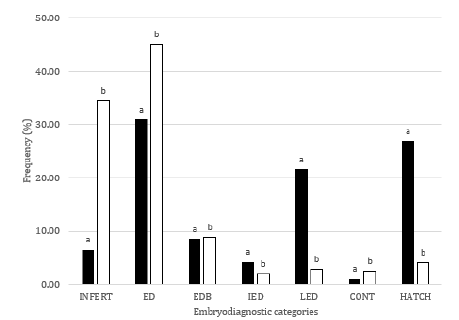
Results of the embryo diagnostic can be observed at Figure 1. The values for the seven categories in the embryo diagnostic were different (P<0.05) between treatments. A higher proportion of the eggs were classified as infertile in T1 (34.5%) comparing to T2 (6.6%). Early embryo mortality (ED + EDB = 54.0%) was also higher in T1 with respect to T2 (ED+EDB = 39.5%), however, ED category contributed with most of the dead embryos in both treatments (45.2 and 31.0% for T1 and T2, respectively). IED category just contributed with a minor proportion of the embryo mortality in T1 (2.0%) and T2 (4.3). LED show a value of 2.8 and 21.6% for T1 and T2, respectively. Contaminated eggs represented 2.5% in T1 and 1.0% in T2. As a result of the values in the previous categories of the embryo diagnostic, hatchability values of 4.2 y 26.9% were obtained for T1 and T2, respectively (Figure 1).
Figure 1: Embryodiagnostic categories in eggs from Creole chicken breeders fed feed without (T2) or with nicarbazin (0.05%) (T1). INFERT: Infertile; ED: Early Dead embryo with no blood; EDB: Early Dead embryo with Blood; IED: Intermediate Embryonic Death; LED: Late Embryonic Death; CONT: Contaminated egg; HATCH: Hatched eggs.
Discussion
Even though there are previous reports of the negative effect of nicarbazin on incubation parameters of birds Yoder et al. [3] and Avery et al. [10], there is not information with respect to birds that are not raised under industrial condition environments, as the Creole chickens of Mexico. Results of the current investigation agree with those of Hughes et al. [11] and Jones et al. [12] who found reduced hatchability in eggs from birds exposed to nicarbazin. As stated by Chapman [13], affection of the integrity of the vitelline membrane is the mechanism by which the coccidiostat affects the correct development of the chick embryo, so that early mortality is expected. On the other hand, given what is known about its mechanism of action Yoder et al. [3] the first stages of development are the most negatively affected by nicarbazin. In line with this fact, our results agree with Ott et al. [1] who found a 3.8-fold increase in early embryo mortality when White Rock breeder hens where fed nicarbazin. So that, embryos without blood spots or blood vessels (ED category in the current study) were the most abundant in the embryo diagnostic.
As previously stated, Creole chickens of Mexico are raised under certain degree of harsh environmental conditions in terms of feed, housing, and potential pathogen agents Flota B et al. [14] and Mata E [15] as coccidia. So that it was considered the necessity of evaluate the effect of a coccidiostat on incubation parameters of this poultry genotype. The results obtained indicate that nicarbazin should not be used in the feed of Creole breeder hens, given its negative effect on embryo mortality, specifically on the earliest stages of development.
Conclusion
Nicarbazin negatively affects the incubation variables, mainly in the early embryo mortality and hatchability, of Creole hens of Mexico.
Acknowledgement
Matus Aragón MA and Zárate Contreras D, thank to National Council of Science and Technology (CONACyT, Mexico) for the financial support for their graduates studies.
Conflict of Interest
The authors declare that there is not financial or conflict of interest in this contribution.
References
- Ott WH, Kuna S, Porter CC, Cuckler AC, Fogg DE (1956) Biological studies on nicarbazin, a new anticoccidial agent. Poult Sci 35(6): 1355-1367.
- Dougald LR, Fitz CS (2013) Protozoal infections. In Diseases of Poultry In: David ES (Ed.), (13th edn) Agricultural Research Service, USA pp. 1147-1201.
- Yoder CA, Graham JK, Miller LA (2006) Molecular effects of nicarbazin on avian reproduction. Poult Sci 85(7): 1285-1293.
- Vázquez G, Pérez PR (2000) Estimated gasometric values for the main populations and sites at higher altitude in Mexico. Rev Inst Nal Enf Resp 13(1): 6-13.
- Aviagen (2010) Reproduction-ROSS TECH investigation of incubation practices: Fertility evaluation. Midlothian, UK, p.48.
- Hamburger V, Hamilton H (1951) A series of normal stages in the development of the chick embryo. J Morphol 88(1): 49-92.
- Scott TA, Mackenzie CJ (1993) Incidence and classification of early embryonic mortality in broiler breeder chickens. Br Poult Sci 34(3): 459-470.
- Bellairs R, Osmond M (2014) Atlas of chick development. (3rd edn), United Kingdom, p. 692.
- (2011) SAS Institute Inc. Base SAS®3 Procedures guide. USA.
- Avery ML, Keacher KL, Tillman EA (2008) Nicarbazin bait reduces reproduction by pigeons (Columba livia). Wildlife Res 35: 80-85.
- Hughes BL, Jones JE, Toler JE, Solis J, Castaldo DJ (1991) Effects of exposing broiler breeders to nicarbazin contaminated feed. Poult Sci 70(3): 476-482.
- Jones JE, Solis J, Hughes BL, Castaldo DJ, Toler JE (1990) Reproduction responses of broiler-breeders to anticoccidial agents. Poult Sci 69(1): 27-36.
- Chapman DH (1994) A review of the biological activity of the anticoccidial drug nicarbazin and its application for the control of coccidiosis in poultry. Poult Sci Rev 5(4): 231-243.
- Flota BC, Ramírez MM, Dorantes JJ, José GG, Bautista O, et al. (2016) Description and diversity of family plots in rural areas of Campeche in Mé Agroproductividad 9: 38-43.
- Mata EA (2018) Phenotypic characterization and productive behavior of backyard chickens in the state of Campeche. Mé
© 2019 González Cerón F. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






