- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Morphological Study of the Reproductive Organs of Prepubertal Gilts
Jackson Brendo Gomes Dantas1, Regina Lúcia dos Santos Silva1, Liliane Moreira Silva Gomes2, Luciana Rocha Faustino3, Leonardo Atta Farias1, Guilherme José Bolzani de Campos Ferreira1 and Cleidson Manoel Gomes da Silva4*
1 Department of Animal Science, Brazil
2 Federal Institute of Pará, Brazil
3 Biotechnology Graduate Program, Brazil
4 Institute of studies of the humid tropics, Brazil
*Corresponding author:Cleidson Manoel Gomes da Silva, Institute of studies of the humid tropics, Brazil
Submission: June 07, 2019;Published: July 03, 2019

ISSN: 2576-9162 Volume6 Issue2
Abstract
The objective of this study was to characterize the reproductive organs of prepubertal gilts through morphometric analyzes. To perform these analyzes, the reproductive organs of eight prepubertal gilts (n=8) were collected and dissected immediately after slaughter. The morphometric evaluation revealed no significant differences between antimeres (left vs right). Likewise, in the histological evaluation no significant difference was found in the height of the epithelium of the reproductive organs evaluated. In addition, it was possible to characterize the predominant epithelium of the collected parts of the uterine tubes, horns and body. In the uterine tubes the predominantly type of epithelium identified was the simple ciliated columnar, whereas in the uterine body and horns the pseudostratified ciliated epithelium predominates. The diameter of the endometrial glands measured from the uterine body and horns samples did not differ significantly (p>0.05). On the other hand, the follicular diameter differed significantly among the follicular categories evaluated (p<0.01): primordial (24.88±0.22μm), primary (29.65±0.47μm) and secondary (113.96±7.29μm). In conclusion, the reproductive organs of prepubertal gilts did not show significant differences in the analyzed morphometrical parameters. However, the values obtained in this study can be used as physiological values for prepubertal gilts.
Keywords: Swine; morphometry; Histomorphometry; Reproduction
Introduction
Swine can be found on all continents and are commercially seen as highly attractive animals, since they are the most widely consumed meat in the world. This fact has greatly stimulated the development and/or the improvement of reproductive technologies, aiming to increase the efficiency of swine production [1]. However, successful implementation of these technologies has been limited because of several exogenous (thermal environment, nutrition, season, stress) and endogenous factors (body composition and body weight, immunological status, growth rate and health condition) [2,3]. In addition, the co-existence of congenital or acquired changes present in the reproductive organs of gilts and sows affects dramatically the future fertility of the female, which increases the production costs [4].
Studies by many researchers indicate that the developmental anomalies in the reproductive system of sows are difficult to identify and are rarely diagnosed based on clinical examination [5]. Thus, post-slaughter examination of the reproductive organs of animals raised in particular farm conditions may be highly useful in breeding practices. An earlier study [3] reported that reproductive disorders are one of the major problems in modern pig production and represent the major reason for culling sows. Therefore, the morphometrical evaluation of the reproductive system of gilts before reaching sexual maturity could be a useful indicator for the estimation of potential fertility in sows [4]. Furthermore, another important indicator of the reproductive capacity of a species is the reserve of preantral follicles in the ovaries [6].
At this moment, a limited number of studies has reported histomorphometric characteristics of the reproductive organs of pigs before reaching sexual maturity. Therefore, the evaluation of macroscopic and microscopic morphology of the reproductive organs is necessary and will be useful for the development of future technologies to maintain or improve the fertility in sows. Thus, the purpose of this study was to characterize the reproductive organs of prepubertal gilts through morphometric analyzes.
Material and Methods
The present study was performed according to the principles of the Animal Experimentation Ethics Committee of Federal University of Piauí (protocol number: 184/16). Immediately after slaughter, the reproductive tract from eight prepubertal gilts (n=8), body weight varying from 40 to 50Kg, was completed removed and transported to the Laboratory in saline solution (0.9%) at 4 ºC.
In the laboratory, the weight and length of the uterine body, horns and tubes were measured using a digital balance (accuracy up to 0.1g) and metric tape (Cateb®), respectively. For histological examination cross-sections were performed in each organ to produce fragments of approximately 0.5cm in length, ensuring that all tissue layers were present. All samples were fixed in 10% buffered formalin for 24 hours. Then, the fragments were maintained in 50% ethanol solution until histological processing (dehydration, clarification and paraffin embedding), which was followed by microtomy (4 μm) and HE staining.
Regarding the ovaries, they were dissected, and their smallest and largest diameters were measured using a digital caliper (accuracy up to 0.01mm; Pantec®), with their weights being measured as previously described. Ovarian volume (cm3) was determined by immersing each ovary in a calibrated measuring cylinder containing a predetermined volume of physiological solution. Then, each ovary was divided in half and immediately fixed in Carnoy`s solution for 18 hours. After fixation, each hemi-ovary was submitted to histological procedures, then cut into 7um sections. Each section was mounted on glass slides and stained with HE.
Preantral follicles were classified according to Rodgers [7], i.e, primordial follicles (oocyte surrounded by a single layer of flattened granulosa cells with ellipsoidal shape), primary follicles (oocyte surrounded by a single layer of cuboidal granulosa cells with rounded shape) and secondary follicles (oocyte surrounded by one or more layers of cuboidal granulosa cells with cuboidal shape).
All histological sections were examined using a trinocular optical microscope (Nova Optical Systems®, São Paulo, Brazil), equipped with a digital camera (TOPCAM™ 5 Megapixel) for photographic recording of images. Three slides of each organ were made per animal. Then, 10 randomized fields were focused on each slide to identify the type of epithelial tissue and to measure its height (40x objective) and to measure the muscle layer thickness and the glandular diameter (objective 20x). The diameter of the preantral follicles was obtained only when the oocyte nucleus was visible (objective 40x). All measurements were performed using ToupView® 3.7 software.
For each analyzed variable (length and weight of the uterine body, horns and tubes; largest and smallest diameter, weight and volume of the ovaries), data were submitted to normality (Kolmogorov-Smirnov test) and homogeneity of variances (Levene test) analyzes. The placement of reproductive organs (left × right antimere) and follicular diameter were subjected to an analysis of variance (ANOVA). Due to the heterogeneity of the variances, height of the epithelium, muscle layer thickness and endometrial glands diameter were compared using the Kruskal-Wallis non-parametric test. Results were expressed as the mean±SD and differences were considered to be significant when p<0.05.
Results and Discussion
In the present study, no signs of ovulation were observed in any ovary, nor the presence of luteal or albicans bodies, confirming that all experimental animals had not yet ovulated, i.e., all females were prepubertal. This condition is particularly desirable for morphometric studies, since the reproductive organs are all at the same maturation level, having been exposed to similar physiological hormonal conditions. Therefore, data obtained under these circumstances are highly relevant for the understanding the reproductive physiology of future matrices and may serve as parameters for the application of assisted reproduction techniques in these animals.
The results on the morphometry of prepubertal gilt reproductive organs are presented in Table 1. The morphometric analysis did not show significant differences between antimeres (left vs right) in none of the evaluated parameters. Similar results were obtained by Oberlender [8] and Monteiro [9]. These authors reported the absence of significant morphometric differences between the reproductive organs from different antimeres (left vs right) in female young sows and cows, respectively.
Table 1:Morphometric data of the reproductive organs of prepubertal gilts and comparison between left and right antimere.
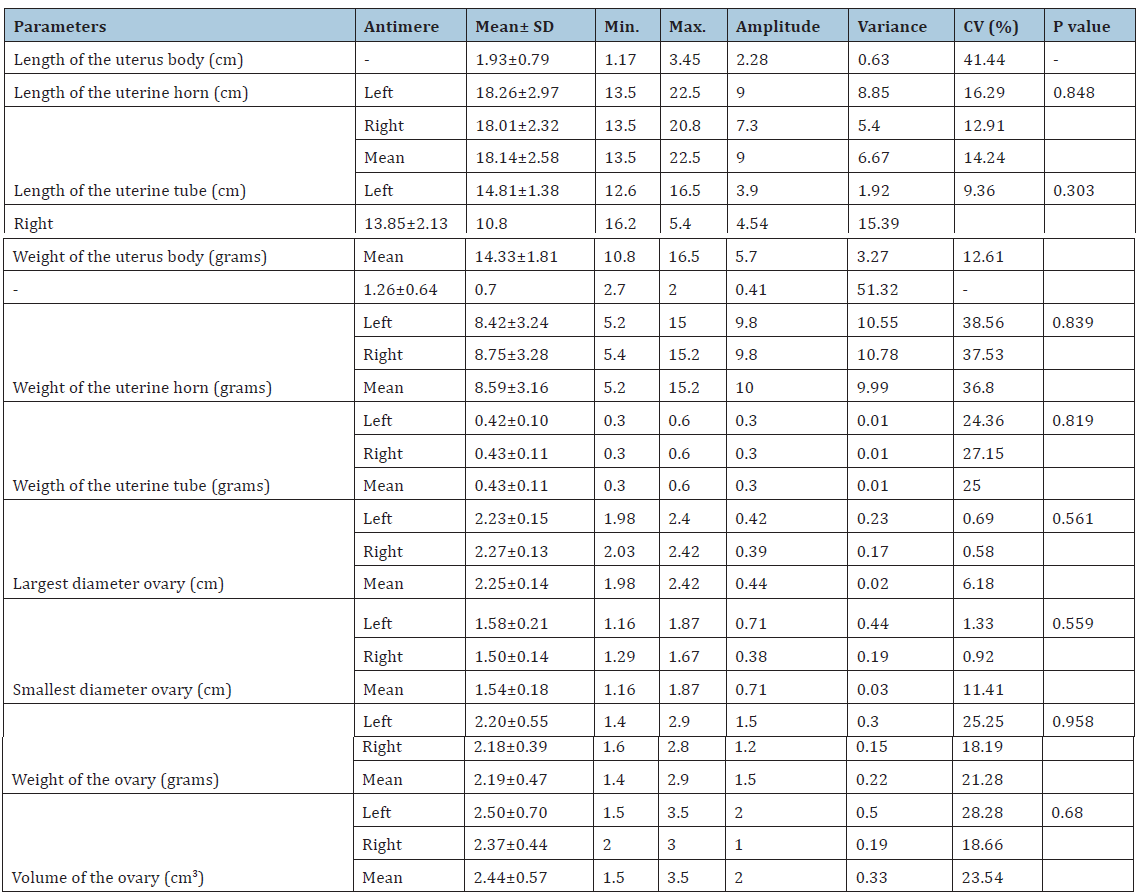
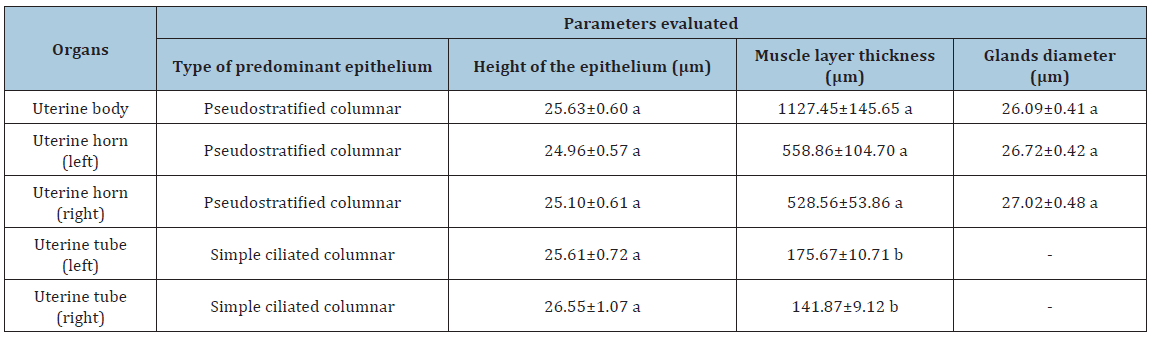
Table 2:Histomorphometric evaluation of the reproductive organs of prepubertal gilts.
In the present study, the length and weight of uterine bodies, horns and tubes of prepurbetal gilts were smaller than those observed in previous studies. According to Oberlender [8] lengths of uterine horns and tubes varying from 32.5 to 96.5cm and from 16.5 to 26.8cm, respectively. These same authors related that weights of uterine horns and tubes varied from 15.7 to 72.9 g and from 0.6 to 1.7g, respectively. In another study, Guimarães [10] working with pigs of the Landrace race, reported uterine body lengths from 4.5 to 8.2cm, uterine horns with 77.2–130.8cm and uterine tubes varying from 23.7 to 45.9cm. In the Textbook of Veterinary Anatomy, Dyce [11] described that swine uterine body have approximately 5cm in length, each uterine horn measures about 1m in non-pregnant animals, uterine tubes have about 20cm and the ovaries length reach 5 cm. From these studies, it becomes evident that there are great discrepancies in the literature regarding the morphometry of swine reproductive organs. These contradictions are possibly related to animal age, weight, race, bodyweight gain rate and nutritional and management conditions, which result in variations in the measured parameters, hindering comparisons among different studies.
The results for the ovarian morphometry were similar to those obtained by Bagg [12], which reported an average size of 2.41cm for the largest and 1.59cm for the smallest ovarian diameter. On the other hand, the ovarian weight and volume observed herein were smaller than previously reported [4,8]. These differences may be related to the studied animal category. In the present study, all animals were sexually immature, with bodyweights ranging from 40 to 50kg, when the reproductive organs are still being fully developed [5]. According to Tummaruk [13], gilt bodyweight and growth rate are positively correlated to the size of reproductive organs. These authors estimate that for each 10kg of gilt bodyweight, there is an increase of 67g and 21cm in the uterus.
There were no significant differences in the height of the epithelium of different organs. There were, however, different types of covering epithelium (Figure 1). In uterine bodies and horns the predominant kind of epithelium was the pseudostratified columnar, with few areas presenting ciliated-epithelium, probably following a uterine tube/horn transition, which rests on a proper layer composed by non-modeled dense connective tissue, as described previously [14].
Figure 1:Characterization of the epithelium of the uterine tubes, horns and body of prepubertal litter. A) Simple ciliated columnar epithelium. B) Pseudostratified columnar epithelium. C) Pseudostratified columnar epithelium. EP: Covering Epithelium; LP: Lamina Propria
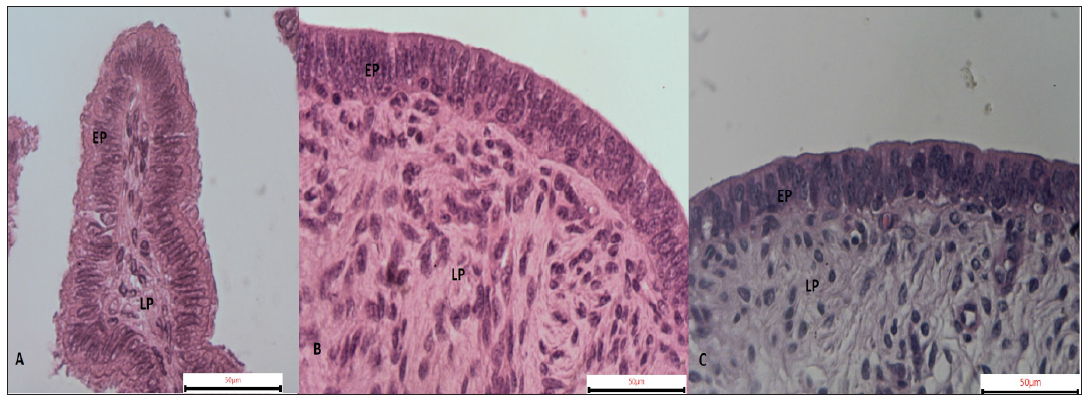
In the uterine tubes the epithelium was characterized as simple ciliated columnar resting on a lamina propria of loose connective tissue (Figure 1). Similar observations were made by Sant'Ana [15] who noted that the mucosa of the uterine tube of adult sows is covered by simple columnar epithelium formed essentially by ciliated cells. In another study, Monteiro [9] reported that the mucosa of the uterine tubes of prepubertal gilts presents areas of simple ciliated columnar epithelium.
The muscular layer thickness of the uterine tubes was significantly inferior (p <0.05) compared to those from the other reproductive organs evaluated. These differences can be justified by the anatomical function that each organ exerts. The cervix and uterine horns actively participate in the delivery mechanism, in order to expel the fetus from the uterine environment [16]. On the other hand, the uterine tubes are responsible for gametes transportation, sperm capacitation, oocyte fertilization, embryo development, as well as embryo migration to the implantation site in the uterus [17].
In this study, no significant histological differences were found between the right and left uterine horns. Both uterine horns had a glandular mucosa without prominent folds, followed by a layer of loose connective tissue. In addition, in the muscular layer were evidenced a well difined inner circular layer and an outer longitudinal layer of smooth muscle, with few areas of cell accumulation. Similar results were described by Monteiro [9] who also did not find significant histological variations between the right and left uterine hornswhen studying the morphology of uterine horns of cows and heifers.
The diameter of the glands measured from cervical and uterine horn samples did not vary significantly. In a previous study, Oberlender [8] reported that the mean diameter of gilt uterine glands may vary from 3.63 to 39.89 μm. Therefore, the results found in the present study are in agreement with the expected for this species.
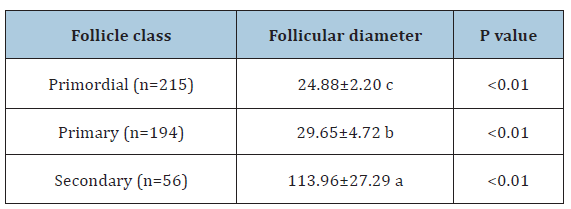
The folicular diameter differed significantly (p<0.01) among the evaluated folicular categories (Table 3). Similar results were reported by Silva [6], who observed significant differences after comparing the diameter of primordial, primary and secondary follicles in swines. In general, the histological characteristics of swine preantral follicles are similar to those of other.
Table 3:Mean diameters of preantral follicles (± SD) from prepubertal gilts.
Conclusion
Taken together, our results showed thatreproductive organs of prepubertal gilts did not show significant differences in the analyzed morphometrical parameters. However, the values obtained in this study can be used as physiological values for prepubertal gilts. In addition, studies of this kind may be used as a basis for the application of assisted reproduction techniques in these animals, as well as for comparison with females of other species.
References
7. Rodgers RJ, Irving-Rodgers HF (2010) Morphological classification of bovine ovarian follicles. Reproduction 139(2): 309-318.
9. Monteiro CMR, Farias EC, Perri SHV, Souza, WM (2003) Estudo das características histológicas do útero e tubas uterinas de vacas e novilhas da raça Nelore (Bos primigenius indicus). Brazilian Journal of Veterinary Research and Animal Science 40(1): 45-54.
11. Dyce KM, Sack WO, Wensing CJG (2010) Tratado de Anatomia Veterinária. In: Pelve e órgãos reprodutivos do suíno. (4th edn), Elsevier, Rio de Janeiro, Brazil, pp. 772-779.
16. Hafez ESE, Hafez B (2004) Reprodução Animal. In: Hafez B, Hafez ESE (Eds.), Anatomia da reprodução feminina. (7th edn), Manole, São Paulo, Brazil, pp. 13-39.
17. Ozen A, Ergün E, Kürüm A (2010) Histomorphology of the oviduct epithelium in the Angora rabbit. Turkish Journal of Veterinary and Animal 34(3): 219-226.
© 2019 Cleidson Manoel Gomes da Silva. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






