- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Campylobacter in Poultry: Species Emergence, Pathogenesis and Antibiotic-Resistance Prevalence
Ahmed Marroki* and Bousmaha ML
Department of Biology, Algeria
*Corresponding author:Ahmed Marroki, Department of Biology, Algeria
Submission: September 10, 2018;Published: February 13, 2019

ISSN: 2576-9162 Volume5 Issue5
Abstract
Members of the genus Campylobacter are Gram-negative bacteria, microaerophilic which colonize the mucosal surfaces of the intestinal tracts of humans and animals. Many Campylobacter species belonging to this group are known pathogens in humans and animals and they are one of the most commonly reported causes of bacterial gastroenteritis in the worldwide. The aim of this review is to describe the principals physiological, biochemical traits of genus and the methods used for isolation, identification and detection of Campylobacter species and related organisms from food, and primarily poultry products. The relationship between the emergence and prevalence of antibiotic-resistance of Campylobacter spp., strains found in poultry problems in developed and developing countries and possible transmission to humans are also analyzed.
keywordsCampylobacter; Emergence; Prevalence; Antimicrobial-Resistant; Poultry; Gastroenteritis; Identification
Introduction
The Campylobacter species are Gram-negative spiral, rod-shaped, or curved bacteria with a single polar flagellum, bipolar flagella, or no flagellum, depending on the species and microaerophilic [1]. The genus Campylobacter initially classified as Vibrio spp. due to their spiral morphology was first proposed by [2]. Member of this genus colonize the mucosal surfaces of the intestinal tracts, oral cavities, or urogenital tracts of a wide range of bird and animal hosts. Campylobacter spp. Are normal intestinal inhabitants of a wide variety of animals and avian species but frequently pathogens of humans. The members of Campylobacter are considered responsible for human bacterial enteritis and poultry meat is recognized as a primary source of infection [3]. The infection is most often acquired through consumption of contaminated food, in particular poultry products, however, other sources also contribute to human infections [4]. The incidence and prevalence of Campylobacteriosis (Campylobacteriosis is an enteric infection caused by members of the genus Campylobacter [5,6]) have increased in both developed and developing countries over the past 10 years and considered as the most commonly reported zoonotic bacterial disease within the European Union.
Many species of domestic poultry such as chickens, turkeys, ducks, geese, and wild birds are frequently infected with thermophilic Campylobacter, primarily C. jejuni [7]. Poultry products are considered one of the most important sources of protein for humans, especially in developing countries. In poultry, mainly in broiler chickens, C. jejuni is the predominant species colonizing the flocks, followed by C. coli and rarely other species. Also, the ability to survive in low temperatures explains why refrigerated carcasses of poultry contaminated in the slaughter process are a common source of C. jejuni infections [8].Today, numerous antibiotics are used for the treatment of infectious diseases. Some antibiotics agents are effective against only a limited range of infections bacteria (narrow spectrum); others are effective against a wider range (broad spectrum) [8]. The treatment with antibiotics has been shown to be beneficial in patients wit gastroenteritis or other extra-gastrointestinal infections caused by emerging Campylobacter spp. However, the emergence of antibiotic-resistant Campylobacter constitutes a serious threat to public health. With the emergence of antibiotic-resistant Campylobacter constitutes a serious problem in public health with significant social, medical and economic consequences.
The aim of this review was describes:
A. Taxonomy of Campylobacter genus including physiological, biochemical and molecular properties;
B. The principals medium and methods used for identification of Campylobacter species from food, poultry products and stool samples;
C. The most common human infections caused by the pathogenic species of Campylobacter
D. The relationship between the emergence and prevalence of antibiotic-resistance of Campylobacter spp. Strains found in poultry problems in developed and developing countries and possible transmission to humans.
Taxonomy and characteristics of Campylobacter spp
The early history of the genus Campylobacter, starting with the first description in 1886 by Theodor Escherich [9], who published series of articles in Münchener Medizinishe Wochenschrift , in which he described spiral-shape bacteria in colon of children of what he called “Cholera infantum”. The first isolation of a Vibrio-like organism from aborted ovine fetuses was by [9], who implicated these organisms as causal agents of abortion in sheep. A few years later [10] in the U.S. reported on the association of similar organisms with bovine abortion. Campylobacters were originally referred to as “micro-aerophilic vibrios” due to their spiral morphology [11,12] reported similar Vibrio from the blood cultures of humans with gastroenteritis [2] proposed the genus, Campylobacter (“curved rod” in Greek). They transferred these Vibrio species, V. fetus and V. bubulus into the new genus, Campylobacter, as C. fetus sp. nov., comb. nov., and C. bubulus sp. nov., comb. nov., respectively. However, the microaerophilic vibrios differed significantly from V. cholerae and certain other vibrios and vibrio-like organisms with respect to their biochemical and physiological properties, and DNA base composition analysis.
The genus Campylobacter was first proposed by [2], and included just two species, C. fetus and C. bubulus. Campylobacter genus belongs to the Proteobacteria phylum that consists of over 200 genera and represents the largest and most diverse group of organisms and contains the majority of Gram-negative species. This phylum is divided in subdivisions: Alpha-, Beta-, Gamma-, Delta-, and Epsilonproteo bacteria [13]. The Campylobacter genus belongs to the family Campylobacteraceae, the order Campylobacterales, the class Epsilon proteobacteria, and the phylum Proteobacteria. Since its first description, the genus has grown to include several important human and animal pathogens that are primarily classified through phylogenetic means. The family Campylobacteraceae comprises the genera Campylobacter, Arcobacter, and Sulfur spirillum, with average G+C content of the DNA between 29-47 [14]. The Campylobacter genus consists of a large and diverse group of bacteria, currently comprising more than 30 species and subspecies. The members of genus carry a relatively small genome that is a singular, circular chromosome of 1.59-1.77 MBP in size. All strains showed growth at 25 °C and 37 °C, but no growth at 42 °C. The only other members of the genus Campylobacter showing growth at 25 °C after 72h are C. fetus and C. hyointestinalis subsp. Hyointestinalis [15].
Most C. fetus subsp. fetus, but not C. fetus subsp. venerealis and all C. hyointestinalis strains are able to grow at 42 °C [16], the optimum growth temperature is 30 to 37 °C [17], in microaerobic to aerobic conditions. Strains show no hydrolysis of casein or gelatin; most strains do not hydrolyze urea. Isolated primarily from the reproductive organs and intestinal tract of man and animals [18], Campylobacter spp. are small, non spore forming, Gramnegative bacteria that have a characteristic curved, S-shaped, or spirally curved rods, 0.2 to 0.8μm wide and 0.5 to 5μm long [19], nonsaccharolytic bacteria with microaerobic growth requirements and a low G+C content.. The genus Campylobacter was consisted in 26 species, 2 provisional species, and 9 subspecies, at present, the genus is recovering 28 species [16,20], with C. fetus as the type species [2]. Cells of most species are motile, with a characteristic cork-screw-like motion performed by means of a single polar unsheathed flagellum at one or both ends of the cell. Cells of some species are nonmotile (C. gracilis) or have multiple flagella (C. showae).
Most Campylobacter species have a respiratory type of metabolism and several species (C. concisus, C. curvus, C. rectus, C. mucosalis, C.2m showed, C. gracilis, and, to a certain extent, C. hyointestinalis) require hydrogen or format as an electron donor for microaerobic growth. In addition, several species grow in anaerobic conditions with fumarate or nitrate as electron acceptor; these species grow only in microaerobic conditions if hydrogen, format, or succinate is supplemented as electron source. Oxidase activity is present in all species except C. gracilis. Gelatin, casein, starch, and tyrosine are not hydrolyzed. Typical biochemical characteristics are reduction of fumarate to succinate, negative methyl red reaction and acetoin and indole production; and for most species, reduction of nitrate, and absence of hippurate hydrolysis. Actually, selective media are developed and can be used either for direct plating or for an enrichment step followed by plating for isolation of Campylobacter. Numerous types of enrichment broths have been developed for isolation of Campylobacter from foods [21].
Usually, enrichment broths consist of a basal medium, such as brucella-FBP (a combination of ferrous sulfate, sodium metabisulfite, and sodium pyruvate), or nutrient broth [21,22], modified charcoal cefoperazone deoxycholate, Park and Sanders, Bolton, Hunt and Radle, and Hunt broths, or supplemented with antimicrobials [21-23]. Also, other broth medium has been developed such as Campylobacter enrichment broth (CEB). The incorporation in broth medium, the enzyme Oxyrase is particularly effective in reducing the levels of oxygen or using different antibiotics in medium improving the isolation of Campylobacter spp. from naturally samples [24,25]. Many agar medium particularly Preston, Doyle and Roman, charchoal cefoperazone deoxycholate (CCDA), Butzler agars and CAT agar (Modified CCDA) [26], has been tested for isolating the Campylobacter from contaminated samples visible colonies usually appear on the plating media within 24-48h.
Methods of identification and typing of Campylobacter spp.
Members of Campylobacter can be distinguished from other microorganisms on the basis of several standard criteria and can be distinguished from one another on the basis of biochemical testing. But these tests have not found a practical application in laboratories because of their lack of reliability and time-consuming (Table 1 & 2). Today, several methods are used for identification of the strains belonging to the genus of Campylobacter. Among these techniques, the miniaturized most probable number (MMPN) methods, which can be performed quickly, have been developed to facilitate enumeration of several target organisms [27-29].This method has been developed by [30], for the enumeration of thermophilic Campylobacter spp. from critical poultry-associated reservoirs using a modification of blood- free Bolton broth (supplemented with 25mg/l of sulfamethoxazole) and Campy Food ID agar. This method was based on a procedure developed by [29] for the enumeration of Salmonella from poultry matrices. The immunological methods are also currently used for identifying and detection of Campylobacter spp. in stool and food samples. Although, a variety of immunoassays have been developed for testing clinical and food samples for Campylobacter spp.
Table 1:Characteristics differentiating species of the genus Campylobacter according [16].
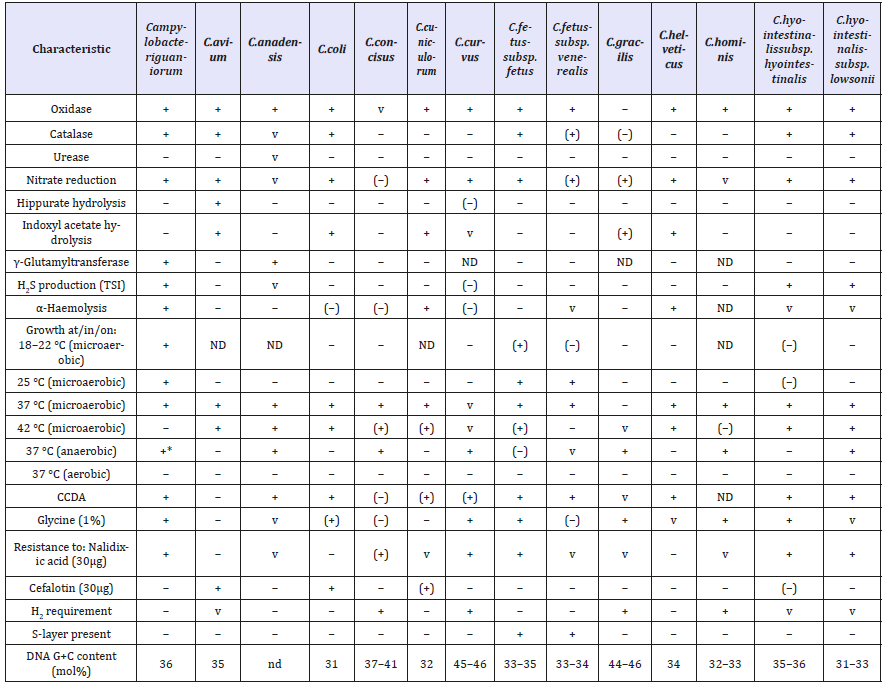
Table 2:Characteristics differentiating species of the genus Campylobacter.
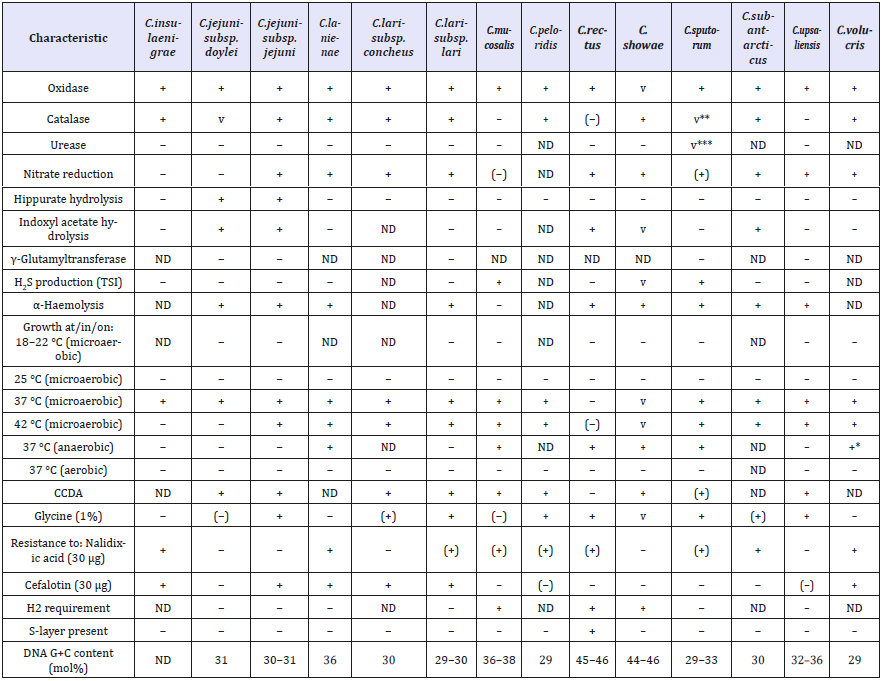
These assays require approval by regulatory bodies. Some of the commercial immunoassays such as (EIAs) are available for culture-independent identification of Campylobacters are also used but have not been extensively validated [30-32]. In the last decade, the VIDAS/MiniVIDAS CAM (bioMerieux, Hazelwood, MO), an automated EIA for detection of thermotolerant C. jejuni, C. coli and C. lari, has received the most attention over [33]. With the immunological tests, more than 60 different serotypes based on somatic (O) antigen and 50 different serotypes based on heatlabile antigens (capsular and flagellar) have been identified [34- 36]. For example, in United States, three immune assays have been tested by Food and Drug Administration (FDA). Each of these tests detects a common Campylobacter surface antigen that is shared by C. jejuni and C. coli, so the immunoassays detect both species of Campylobacter in stool specimens but cannot differentiate them such as: the Prospect Campylobacter Microplate Assay (Remel, Lenexa, KS), the Premier CAMPY (Meridian Bioscience, Cincinnati, OH), and the Immuno Card STAT CAMPY (Meridian Bioscience, Cincinnati, OH) [37].
Molecular methods have an enormous impact on the taxonomy of Campylobacter. Several alternative and rapid methods have been developed for detecting and confirming Campylobacter spp [38,39], those that include fluorescence in situ hybridization (FISH) [40], latex agglutination (commercially available; Microscreen® Campylobacter kit) [41]. However, the most effective confirmation methods for identification of species are those based on the polymerase chain reaction (PCR) assays. The majority of these methods have been multiplexed and incorporated into the few realtime PCR assays, also other test such as the BAX (Dupont, Qualicon, Wilmington, DE, USA) and iQ-Check (BioRad Laboratories, Hercules, CA, USA) require an enrichment of approximately 24-48h, have been used in USA for detection of Campylobacter in poultry carcass with a higher contamination rate. The 16S rRNA, sequencing and DNA-DNA hybridization analysis [35,36,42], can be used for culture confirmation or for direct detection of Campylobacter from environmental or clinical samples. Many DNA-based methods have been also developed for molecular typing of Campylobacter isolates from chickens and other animal reservoirs, these include pulsed-field gel electrophoresis (PFGE) [38,43], random amplified polymorphic DNA-PCR (RAPD -PCR [44-46], and amplified fragment length polymorphism (AFLP) [47,48].
Other methods can be used for detection of Campylobacter such as: the whole-cell protein SDS-PAGE [35,47-50], and matrixassisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry also used for identification, detection of Campylobacter species and related organisms in [51-53].Sequencebased typing methods target the variable region of the fla gene (encoding the flagellin subunit), several house-keeping genes (multilocus sequence typing, MLST) (aspA, glnA, gltA, glyA, pgm, tkt and uncA) [54] or the cmp gene (encoding the major outer membrane protein), rpoB, groEL, hsp60 gene and cpn60 [35,55,56], have made identification of many species of Campylobacter. Recently the methodologies for the isolation of Campylobacter spp. from poultry products especially C. jejuni and C. coli are reviewed by [57].
Campylobacter: habitat & pathogenesis
Campylobacter spp., commensally organisms of poultry is known pathogens in humans and animals and has been recognized as the leading cause of bacterial gastroenteritis worldwide [58]. The Campylobacter are considered as zoonotic bacteria (bacteria they are transmitted from animals to humans and cause disease in humans.) and can spread easily between different animals and between animals and humans. The most prevalent subspecies about human infection are C. jejuni and C. coli, and they are typical and cause disease in humans. zoonotic pathogens. Campylobacter infection can present with a wide range of symptoms from watery diarrhea to dysentery, often accompanied by fever and severe abdominal cramps [1]. In humans, Campylobacter spp. Is one of the major causes of bacterial gastroenteritis, inducing an acute self- limiting diarrheic disease (is the unfavorable outcome of the interactions between the host, pathogen, and environments whereby conditions exist to favor pathogen growth and spread. (Disease is an abnormal state)[59]), inflammatory bowel diseases (IBD), Barrett’s esophagus, and colorectal cancer [1].
Also, it’s implicated in extra-intestinal infections that include bacteremia, hemolytic uremic syndrome, meningitis, and septicemia, lung infections, brain abscesses, and reactive arthritis, in individual cases and small cohorts of patients [1,59,60]. Infection by Campylobacter is associated with the development of Guillain-Barre´ syndrome (GBS), a neurological disorder affecting the peripheral nervous system [61,62]. More recently C. jejuni species has been associated with a rare form of mucosa-associated lymphoid tissue (MALT) lymphoma called immunoproliferative small intestinal disease (IPSID) [63]. Most human infections are caused by the thermophilic species C. jejuni or C. coli. Campylobacter causes an acute gastroenteritis characterized by fever, abdominal pain, and profuse diarrhea that is frequently bloody [64-66]. Most patients recover within 1 week without antimicrobial treatment. Bacteremia and other extraintestinal infections are uncommon complications [67]. Reactive arthritis may occur as a sequel of enteric Campylobacter infections in 1%-5% of patients, and Guillain- Barré syndrome occurs in approximately 0.1% of patients [68,69]. As a foodborne pathogen, the transmission of Campylobacter spp. To humans occurs most commonly by consumption and handling of various kinds of foods of animal origin, with poultry being the most common source [20,54]. Contact with colonized animals or drinking untreated water are potential sources of exposure
The major source of infection today is by consumption of contaminated poultry and unpasteurized milk; person-toperson transmission can occur but is uncommon (Figure 1) [70]. The contamination by these pathogenic bacteria is prevented by thoroughly cooking chicken and pasteuring milk and water treatment.
Figure 1:
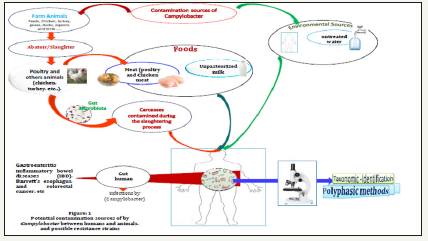
The incubation period ranges from 1-10 days but is most commonly 2-5 days [71]. The duration of fecal shedding can range from 2-7 weeks, although the median duration of shedding is less than 3 weeks [72,73]. Otherwise, the presence of Campylobacter in poultry is generally varied by regions, seasons, and the production stages and types. For example, in the United States, Campylobacter especially C. jejuni is one of the most commonly reported bacterial causes of foodborne infection [74,75] and the European Union [76]. The estimated incidence of Campylobacteriosis in the United States is 13.02 per 100,000 population [77], with an estimated 845,000 cases, 8,463 hospitalizations, and 76 deaths occurring annually, and with 190,566 cases of enteritis being reported in the European Union in 2008 [78].
In developed countries there is a male predisposition, a seasonal peak in cases during the late spring and summer, and a bimodal age distribution with infection being most common in children less than 1 year of age and in young adults from 15-44 years old [65]. In animal, most domestic poultry reared for consumption or egg production, including chicken, turkey, geese, ducks, pigeons, and even ostriches, as well as wild birds, are frequently infected and colonized with Campylobacter species and considered as natural host of these animals. Many of them (these animals) harbor C. jejuni in their intestinal tracts [5,79]. Many vectors are described as origin of contamination which is infected chicken carcasses and meat by Campylobacter and constitute a risk to consumers. The important most frequently are handling and cross contamination in the kitchen, consumption of chicken meat and during the slaughtering process [80-83]. Worldwide, an average prevalence of Campylobacter contamination on poultry carcasses is reported to be in the range of 60-80% [84,85].
In the past decade, a growing number of Campylobacter species other than C. jejuni and C. coli have been recognized as emergence humans and animal’s pathogens in humans [1]. The emerging (species: a term used to describe their underappreciated roles in human and animal diseases) Campylobacter species are likely to contribute to the etiology of gastroenteritis, especially in cases which have no known association with other established pathogens [86-88]). C. concisus, C. ureolyticus, C. upsaliensis, and C. lari, C. hyointestinalis, (C. coli, C. concisus, C. fetus, C. rectus and C. upsaliensis [1]. The Colonization of broiler chickens is common, and contaminated poultry meat is considered to be the most important source of human infections [82]. In association with food or water contaminated, Campylobacters, enter the host intestine and colonize the distal ileum and colon. Following colonization of the mucus and adhesion to intestinal cell surfaces, Campylobacters perturb the absorptive capacity of the intestine by damaging epithelial cell function either directly, by cell invasion or the production of toxin(s), or indirectly, following the initiation of an inflammatory response [89]. Many bacterial factors contribute to the colonization of Campylobacter in poultry [1].
These include flagella, DnaJ (heat shock protein), CiaB (Campylobacter invasin antigen B), PldA (phospholipase A), CadF (Campylobacter adhesin to fibronectin), CmeABC (multidrug efflux pump), CmeR (a pleiotropic regulator), MCP (a methyl-accepting chemotaxis protein), RpoN (sigma factor), the Kps locus (capsule biosynthesis proteins), the Pgl locus (protein glycosylation system), SOD (superoxide dismutase), Fur (ferric uptake regulator), an unnamed lipoprotein encoding gene, FucP (a fucose permease), and CbrR (a bile resistance response regulator [90]. As with other enter pathogens, motility, chemotaxis, adherence, invasion, and toxin production have been recognized as virulence properties [91]. The mechanisms involved by Campylobacter species to persist and colonize the gastrointestinal tract of the host or to spread to systemic sites are shown in (Figure 2). Campylobacter is a mucosal-associated bacterium that has the ability to cross the mucus layer that covers the intestinal epithelium. Some emerging Campylobacter species (C. coli, C. concisus, C. fetus, C. rectus and C. upsaliensis) have been shown to bind and invade intestinal epithelial cells. Investigations suggest that these bacteria can move through the intestinal epithelium either transcellularly (C. fetus) and/or paracellularly by breaking down the tight junctions associated with the barrier (C. concisus).
In addition, tests have shown that emerging species could release effectors proteins into epithelial cells of the host via a presumed T4SS. The S-layer induces resistance to phagocytosis and serum destruction, possibly by inhibiting the stable deposition of complement on the surface of bacterial cells during the systemic phase of infection. Emerging species can also generate a number of toxins, including tripartite cytolase distension toxin. Binding of this toxin to the surface of the host cell is provided by CdtA and CdtC, followed by delivery of CdtB into the host nucleus, which triggers cell cycle arrest and DNA damage. Other toxins include cell-bound and secreted haemolysins have the ability to lyse red blood cells (C. coli and C. concisus) [1].
Prevalence of antimicrobial-resistant Campylobacter
The emergence of antibiotic-resistant bacteria is a problem with significant social, medical and economic consequences. There are two distinct stages in the emergence of antibiotic-resistant bacteria: the genetic change (mutation or gene acquisition); and amplification and enrichment of resistant bacteria by exposure to antibiotics (antibiotic selection) [7]. However, antibiotic (are chemical compounds which are produced by various microorganisms (bacteria, fungi, etc.) which suppress the growth of bacteria or synthesized in laboratories. [7]) used in animals is a potential problem for human medicine because antibiotic resistant bacteria can pass through the food chain to people [7,92]. The antibiotics were first used in veterinary medicine for the treatment of mastitis in dairy cows [93]. Generally, many bacteria have evolved various resistance mechanisms to help them survive the chemical onslaught of antibiotic treatment.
These mechanisms include prevention of drug entry into the cell, as illustrated by Gram-negative bacteria whose outer lipid layer serves as a barrier to certain antibiotics. In general, bacterial resistance to antimicrobials occurs via either mutations in chromosomal loci or acquisition of horizontally transferred mobile genetic elements such as plasmids, phages, transposons and integrons [94-97] indicate that antibiotics generally work by one of five mechanisms:
A. Inhibition of bacterial cell wall synthesis (penicillin’s, cephalosporins, bacitracin, vancomycin)
B. Inhibition of protein synthesis (chloramphenicol, erythromycin, streptomycin, tetracyclines)
C. Inhibition of essential metabolites (sulfanilamide and trimethoprim prevent folic acid synthesis)
D. Injury to plasma membrane (polymyxin b, nystatin, miconazole)
E. Inhibition of nucleic acid replication and transcription (quinolones, rifampin)
Today, the use of antimicrobial agents to promote growth and control diseases in food animals has resulted in the emergence and dissemination of antimicrobial-resistant bacteria, including antimicrobial- resistant Campylobacter. The emergence of antibiotic resistant strains demonstrates that the treatment of bacterial infections cannot rely on the use of antibiotics without some critical consideration because these can contribute to the problems with antibiotic resistance in humans [98]. Many studies have reported the prevalence of antimicrobial-resistant Campylobacter in animal reservoirs and poultry in different countries [59,99-103].In the European Union report for 2012 [104] the C. jejuni isolates from broilers exhibit high resistance to ciprofloxacin (57.2%), nalidixic acid (55.5%) and tetracycline (40.6%), and low resistance to erythromycin (1.6%) and gentamicin (0.9%). Higher resistance was exhibited by the broiler isolates to ciprofloxacin (76.6%), nalidixic acid (70.2%) and tetracycline (74.6%), and moderate to low resistance to erythromycin (15.5%) and gentamicin (3.8%). In general in the culture-confirmed cases of Campylobacter, the main treatments given are macrolides [105].
The prevalence of antibiotic resistance in Campylobacter isolates from commercial poultry was tested for their susceptibility of antibiotics in suppliers in, South Africa by Bester and Essack [106]. The result obtained show that multi resistance was detected in 23% and 43% of the isolates from broiler and layer chickens, respectively. The prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China was conducted by [107]. In this study minimal inhibitory concentrations of 11 antimicrobial agents were determined using the agar dilution method recommended by CLSI against 275 Campylobacter. The result obtained show that 98% of the tested Campylobacter isolates were resistant to quinolones and tetracyclines. The C. jejuni isolates exhibited a high rate of resistance to phenicol antibiotics. On the contrary, the C. coli isolates showed a high-level resistance to macrolides and gentamicin. The authors conclude that antimicrobial resistance is highly prevalent in the poultry Campylobacter isolates from China, and many of them are resistant to multiple antimicrobial agents with high MIC values. A study conducted by [108], evaluate the prevalence and antimicrobial susceptibility of Campylobacter spp. isolated from different chicken production systems.
The result obtained by author’s show that the prevalence of resistance of Campylobacter isolated from all origins was 80 to 90% for the fluoroquinolones studied. Also, a high resistance of tetracycline occurrence was also found for the Campylobacter spp. tested (58% for C. jejuni and 76% for C. coli) [109], screened antimicrobial susceptibility of 145 Campylobacter strains from different origins in Italy. The result obtained by authors revealed a high level of resistance for ciprofloxacin (62.76%), tetracycline (55.86%) and nalidixic acid (55.17%). Another study by [110], investigate the prevalence of Campylobacter spp. In fresh chicken meat samples collected from different farms the meat of free-range in North Western Greece and assess the respective antimicrobial susceptibility of the isolates. The prevalence of Campylobacter isolation was in average 28.73% and ranged from 18.51% to 50%. Therefore the prevalence reported in this study is quite close to the average prevalence of the European Union (29.6%).
Nevertheless, when comparing the reported prevalence of Campylobacter spp. among the Mediterranean countries (Spain, Italy, France and Greece) during 2005-2010, the results obtained are considerably lower. A high resistance rates are reported by this study in relation to ampicillin, tetracycline, ciprofloxacin and erythromycin. The practices and factors influencing the use of antibiotics in selected poultry farms in Ghana was reported by [111]. This study investigates the use of essential antibiotics in poultry production in 400 farms in Ghana and assesses factors influencing farmers’ choice of antibiotics for use on their farms. Farmers reported the use of 35 different antimicrobial agents for management of conditions such as Newcastle, fowl pox, coccidiosis, and coryza. From these agents, 20 essential antibiotics belonging to 10 antibiotic classes were extracted. Frequently employed antibiotics were tetracyclines (24.17%), aminoglycosides (17.87%), penicillin’s (16.51%) and fluoroquinolones (10.55%). Only 63% of the farms completed recommended antibiotic course durations, 58% reported following recommended withdrawal periods and 88% sought veterinary advice before administration of antibiotics. The use of antibiotic-containing agents was observed to be dependent on internal factors such as size, presence of other livestock on the farm and infections. External factors such as easy access to antibiotics also influenced farmers’ use of antibiotics.
These findings call for stricter regulations on access to and use of antibiotics on poultry farms in Ghana. Another study was carried out to assess the chemicals and veterinary drugs used, and their possible occurrence as residue in poultry products in randomly selected poultry farms in Ethiopia through questionnaire and observation. The result of this study showed that antibiotics, mainly Oxytetracycline, amoxicillin, cipro floxacillin, and sulfa drugs were used in 100%, 71.4%, 28.6%, and 28.6% of poultry farms, respectively. The study also revealed that piperazine was a common anthelmintic used in 31.0% of poultry farms. The disinfectants, such as, hydrogen peroxide (83.3%), sodium hydroxide (66.7%), and formalin (19.0%) were commonly used in poultry farms though. Among the rodenticides used in farms, zinc phosphide was used more in poultry farms (33.3%). We conclude that there are high possibility of drug and chemical residues occurrence in poultry in the area. A recent study was conducted to determine the prevalence and antimicrobial resistance pattern of Campylobacter isolated from meat, of three different food animal species sold at retail shops in, Pakistan.
The result obtained show that from 125 Campylobacter isolated and tested for antimicrobial resistance against commonly used antibiotics in veterinary and human medicine, 46% isolated from chicken meat, have a highest resistance against enrofloxacin, tylosin, amoxicillin and (80%) were resistant to both ciprofloxacin and colistin. Also, most of the isolates tested (90.4%) were resistant to more than two antibiotics and were considered as multi-drug resistant bacteria. The results obtained in this study concluded that antibiotic resistant bacteria are prevalent in animal meat in Pakistan probably due to uncontrolled use of antibiotics in food animals.
Conclusion
Campylobacter is among the major causes of food-borne illness worldwide and considered the principal cause of gastroenteritis in human. However, the contaminated food especially poultry meat by these pathogenic bacteria are considered the principal cause of an emergence humans and animal’s pathogens. In poultry farms and in livestock, antibiotics are used for therapy and promotion of growth. The misuse of antibiotic is considered the most important factor selecting for emergence of antibiotic resistance. To minimize this problem, it’s recommended to monitor and control bacterial infections by Campylobacter in food production. Antibiotics should be prudently used by the implementation a strategies and guidelines are required for limiting, controlling and minimized the spread and development of resistant bacteria and the genes that encoded for this resistance especially in poultry farms and livestock.
References
- Man SM (2011) The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8(12): 669-685.
- Sebald M, Veron M (1963) Base DNA content and classification of vibrios. Ann Inst Pasteur 105: 897-910.
- Peyrat MB, Soumet C, Maris P, Sanders P (2008) Phenotypes and genotypes of campylobacter strains isolated after cleaning and disinfection in poultry slaughterhouses. Vet Microbiol 128(3): 313-326.
- Griekspoor P, Engvall EO, Åkerlind B, Olsen B, Waldenström J (2015) Genetic diversity and host associations in Campylobacter jejuni from human cases and broilers in 2000 and 2008. Vet Microbiol 178(1-2): 94-98.
- Sahin O, Kassem II, Shen Z, Lin J, Rajashekara G, et al. (2015) Campylobacter in poultry ecology and potential interventions. Avian Dis 59(2): 185-200.
- Bhaduri S, Cottrell B (2004) Survival of cold-stressed Campylobacter jejuni on ground chicken and chicken skin during frozen storage. Appl Environ Microbiol 70(12): 7103-7109.
- JETACAR (Joint Expert Technical Advisory Committee on Antibiotic Resistance) (1999) The use of antibiotics in food-producing animals: antibiotic-resistant bacteria in animals and humans Joint Expert Advisory Committee on Antibiotic resistance (JETACAR). Commonwealth Department of Health and Aged Care and the Commonwealth Department of Agriculture, Fisheries and Forestry, Canberra, Australia.
- Escherich T (1886) Contributions to the knowledge of the intestinal bacteria III. The occurrence of vibrations in the intestine canal and the strokes of the infants. (Articles adding to the knowledge of intestinal bacteria III. On the existence of Vibrio in the intestines and feces of bacies). Miinch Medecine Wochenscher 33: 815-817.
- Mc Fadyean J, Stockman S (1913) Abortion in sheep. Report of the departmental committee appointed to enquire into epizootic abortion. Great Britain Board of Agriculture and Fisheries. Abortion in sheep, United Kingdom, London, pp. 12-23.
- Smith T, Taylor MS (1919) Some morphological and biological characteristics of the spirilla associated with disease of fetal membranes in cattle. J Exp Med 30(4): 299-311.
- Levy J (1946) A gastro-enteritis outbreak probably due to a bovine strain of Vibrio. Yale J Biol Med 18: 243-258.
- King EO (1957) Human infections with Vibrio fetus and a closely related Vibrio. J Infect Dis 101(2): 119-128.
- Gupta RS (2000) The phylogeny of proteobacteria relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol Rev 24(4): 367-402
- Ebruyne L, Evers D, Vandamme P (2008) Taxonomy of the family Campylobacteraceae. In: Szymanski CM, Blaser MJ (Eds), Campylobacter nachamkin I. ASM Press, Washington, USA.
- On SL, Holmes B, Sackin MJ (1996) A probability matrix for the identification of campylobacters helicobacters and allied taxa. J Appl Bacteriol 8(4): 425-432.
- Gilbert MJ, Kik M, Miller WG, Duim B, Wagenaar JA (2015) Campylobacter iguaniorum sp nov isolated from reptiles. Int J Syst and Evol Microbiol 65(3): 975-982.
- Vandamme P, Dewhirst FE, Paster BJ, ON SLW (1991) Campylobacteraceae. In: Brenner DJ, Krieg NR, Staley JT, (Eds), Bergey’s manual of systematic bacteriology Volume 2: The proteobacteria Part C. The alpha, beta, delta and epsilon proteobacteria , volume 2 , Garrity GM, editor-in-chief (2nd edn), New York, USA.
- Vandamme P, DeLey J (1991) Proposal for a new family Campylobacteraceae. Int J Syst Bacteriol 41(3): 451-455.
- Gilbreath JJ, Willian LC, Merrel DS, Hendrixson DR (2011) Change is good variation in the epsilonproteobacterial genera Campylobacter and Helicobacter. Microbiol Mol Biol Rev 75(1): 84-132.
- Kaakoush NO, Castaño RN, Mitchell HM, Man SM (2015) Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28(3): 687- 719.
- Corry JEL, Post DE, Colin P, Laisney MJ (1995) Culture media for isolation of campylobacters. Int J Food Microbiol 26(1): 43-76.
- Bolton FJ, Robertson L (1982) A selective medium for isolating Campylobacter jejuni/coli. Journal Clinical Pathology 35(4): 462-467.
- Kim SA, Lee YM, Hwang IG, Kang DH, Woo GJ, et al. (2009) Eight enrichment broths for the isolation of Campylobacter jejuni from inoculated suspensions and ground pork. Lett Appl Microbiol 49(5): 620-626.
- Abeyta JRC, Trost PA, Bark DH, Hunt JM, Kaysner CA, et al. (1997) The use of bacterial membrane fractions for the detection of Campylobacter species in shellfish. J Rap Methods Autom Microbiol 5(3): 223-247.
- Kim J, Oh E, Banting GS, Braithwaite S, Chui L, et al. (2016) An improved culture method for selective isolation of Campylobacter jejuni from wastewater. Front Microbiol 7: 1345.
- Corry JEL, Atabay HI (1997) Comparison of the productivity of cefoperazone amphotericin teicoplanin (CAT) agar and modified charcoal cefoperazone deoxycholate (MCCD) agar for various strains of Campylobacter, Arcobacter and Helicobacter pullorum. Int J Food Microbiol 38(2-3): 201-209.
- Jagals P, Grabow WOK, Griesel M, Jagals C (2000) Evaluation of selected membrane filtration and most probable number methods for enumeration of faecal coliforms Escherichia coli and enterococci in environmental waters. Quant Microbiol 2(2): 129-140.
- Line JE, Siragusa GR (2006) Biphasic microtitre method for Campylobacter recovery and enumeration method. Journal of Rapid Methods and Automation in Microbiology 14(2): 182-188.
- Pavic A, Groves PJ, Bailey G, Cox JM (2010) A validated miniaturized MPN method, based on ISO 6579:2002, for the enumeration of salmonella from poultry matrices. Journal of Applied Microbiology 109(1): 25-34.
- Chenu JW, Pavic A, Cox JM (2013) A novel miniaturized most probable number method for the enumeration of Campylobacter spp. from poultry-associated matrices. J Microbiol Methods 93(1): 12-19.
- Hoorfar J, Nielsen EM, Stryhn H, Andersen S (1999) Evaluation of two automated enzyme-immunoassays for detection of thermophilic campylobacters in faecal samples from cattle and swine. J Microbiol Methods 38(1-2): 101-106.
- Borck B, Stryhn H, Ersbøll AK, Pedersen K (2002) Thermophilic Campylobacter spp in Turkey samples: Evaluation of two automated enzyme immunoassays and conventional microbiological techniques. Appl Microbiol 92(3): 574-582.J
- Oyarzabal OA, Battie C (2012) Immunological methods for the detection of Campylobacter spp. Current applications and potential use in biosensors, trends in immunolabelled and related techniques. In: Eltayb A (Ed.), ISBN:978-953-51-0570.
- Franco DA (1988) Campylobacter Species: Considerations for controlling a foodborne pathogen. J Food Prot 51(2): 145-153.
- Debruyne L, On SL, De Brandt E, Vandamme P (2009) Novel Campylobacter lari -like bacteria from humans and molluscs: description of Campylobacter peloridis sp nov Campylobacter lari subsp concheussubsp Nov and Campylobacter lari subsp lari subsp nov. Int J Syst Evol Microbiol 59(Pt 5): 1126-1132.
- Debruyne L, Broman T, Bergström S, Olsen B, On SL, Vand, et al. (2010) Campylobacter volucris sp nov isolated from black-headed gulls (Larus ridibundus). Int J Syst Evol Microbiol 60(8): 1870-1875.
- Fitzgerald C (2015) Campylobacter. Clin Lab Med 35(2): 289-298.
- Wassenaar TM, Newell DG (2000) Genotyping of Campylobacter spp. Appl Environ Microbiol 66(1): 1-9.
- Levin RE (2007) Campylobacter jejuni: A review of its characteristics, pathogenicity, ecology, distribution, subspecies characterization and molecular methods of detection. Food Biotechnology 21(4): 271-347.
- Lehtola MJ, Pitkanen T, Miebach L, Miettinen IT (2006) Survival of Campylobacter jejuni in potable water biofilms: A comparative study with different detection methods. Water Sci Technol 54(3): 57-61.
- Wilma C, Hazeleger RR, Beumer FD, Rombouts FM (1992) The use of latex agglutination tests for determining Campylobacter species. Lett Appl Microbiol 14(4): 181-184.
- Roop PR, Smibert IRM, Johnson JL, Krieg NR (1985) Campylobacter mucosalis (lawson, leaver, pettigrew, and rowland 1981) comb nov: emended description. Int J Syst Bacteriol 35(2): 189-192.
- On SLW , Harrington CS (2001) Evaluation of numerical analysis of PFGE-DNA profiles for differentiating Campylobacter fetus subspecies by comparison with phenotypic, PCR and 16S rDNA sequencing methods. Journal of Applied Microbiology 90(2): 285-293.
- Aarts H, Lith VL, Jacobs RW (1995) Discrepancy between penneer serotyping and polymerase chain reaction fingerprinting of Campylobacter isolates from poultry and other animal sources. Lett Appl Microbiol 20(6): 371-374.
- Nielsen EM, Engberg J, Fussing V, Petersen L, Brogren CH, et al. (2000) Evaluation of phenotypic and genotypic methods for subtyping Campylobacter jejuni isolates from humans, poultry, and cattle. J Clin Microbiol 38(10): 3800-3810.
- Tu ZC, Eisner W, Kreiswirth BN, Blaser MJ (2005) Genetic divergence of Campylobacter fetus strains of mammal and reptile origins. J Clin Microbiol 43(7): 3334-3340
- Duim B, Wassenaar TM, Rigter A, Wagenaar J (1999) High resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl Environ Microbiol 65(6): 2369-2375.
- Duim B, Vandamme PA, Rigter A, Laevens S, Dijkstra JR, et al. (2001) Differentiation of Campylobacter species by AFLP fingerprinting. Microbiology 147(Pt 10): 2729-2737.
- Kokotovic B, On SL (1999) High-resolution genomic fingerprinting of Campylobacter jejuni and Campylobacter coli by analysis of amplified fragment length polymorphisms. FEMS Microbiol Lett 173(1): 77-84.
- Rossi M, Debruyne L, Zanoni RG, Manfreda G, Revez J, et al.(2009) Campylobacter avium sp Nov a hippurate positive species isolated from poultry. Int J Syst Evo Microbiol 59(9): 2364-2369.
- Bessède E, Solecki O, Sifre E, Labadi L, Mégraud F (2011 ) Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Clin Microbiol Infect 17(11): 1735-1739.
- Martiny D, Dediste A, Debruyne L, Vlaes L, Haddou B, et al. (2010) Accuracy of the API campy system, the Vitek 2 Neisseria-Haemophilus card and matrix-assisted laser desorptionionization time-of-flight mass spectrometry for the identification of Campylobacter and related organisms. Clin Microbiol Infect 17(7 ): 1001-1006.
- Koziel M, O’Doherty P, Vandamme P, Corcoran GD, Sleator RD et al. (2014) Campylobacter corcagiensis sp Nov isolated from feces of captive lion-tailed macaques (Macaca silenus). Int J Syst EvolMicrobiol 64(8): 2878-2883.
- Bronowski C, James CE, Winstanley C (2014) Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiology Letters 356(1): 8-19.
- Inglis GD, Hoar BM, Whiteside DP, Morck DW (2007) Campylobacter canadensis sp nov from captive whooping cranes in Canada. Int J Syst Evol Microbiol 57(Pt 11): 2636-2644.
- Zanoni RG, Debruyne L, Rossi M, Revez J, Vandamme P (2009) Campylobacter cuniculorum sp nov from rabbits. Int J Syst Evol Microbiol 59(7): 1666-1671.
- Gharst G, Oyarzabal OA, Hussain SK (2013) Review of current methodologies to isolate and identify Campylobacter spp. from foods. J Microbiol Methods 95(1): 84-92.
- Backert S, Tegtmeyer N, Cróinín TO, Boehm M, Heimesaat MM (2017) Human campylobacteriosis. In: Klein G (Ed.), Campylobacter features, detection, and prevention of foodborne disease. Elsevier Academic San Diego, Cambridge, USA, pp. 1-25.
- Foley S (2012) Campylobacter jejuni. In: Lampel KA, Khaldi AS, Cahill SM (Eds.), Bad bug book. Foodborne pathogenic microorganisms and natural toxins handbook (2nd edn). Washington DC, USA.
- Ge B, Wang F, Sjölund KM, McDermott PF (2013) Antimicrobial resistance in Campylobacter: susceptibility testing methods and resistance trends. J Microbiol Methods 95(1): 57-67.
- Yuki N (2001) Infectious origins of and molecular mimicry in, guillainbarre ´ and fisher syndromes. Lancet Infect Dis 1(1): 29-37.
- Leonard EE, Tompkins LS, Falkow S, Nachamkin I (2004) Comparison of Campylobacter jejuni isolates implicated in Guillain-Barre´ syndrome and strains that cause enteritis by a DNA microarray. Infect Immun 72(2): 1199-1203.
- Lecuit M, Suarez F, Lortholary O (2004) Immunoproliferative small intestinal disease associated with Campylobacter jejuni. Med Sci Paris 20(6): 638-640.
- Blaser MJ (1997) Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis 176(Suppl 2): S103-S105.
- Blaser MJ, Engberg J (2008) Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I, Szymanski CM, Blaser MJ (Eds.), Campylobacter. ASM press, Washington DC, USA, pp. 99-121.
- Allos BM (2001) Campylobacter jejuni infections: Update on emerging issues and trends. Clin Infect Dis 32(8): 1201-1206.
- Blaser MJ, Perez GP, Smith PF, Patton C, Tenover FC, et al. (1986) Extraintestinal Campylobacter jejuni and Campylobacter coli infections: Host factors and strain characteristics. J Infect Dis 153(3): 552-559.
- Tam CC, Rodrigues LC, Petersen I, Islam A, Hayward A, et al. (2006) Incidence of guillain barre syndrome among patients with Campylobacter infection: A general practice research database study. J Infect Dis 194(1): 95-97.
- Pope JE, Krizova AX, Garg H, Thiessen P, JM Ouimet (2007) Campylobacter reactive arthritis: A systematic review. Semin Arthritis Rheum 37(1): 48-55.
- Cheng HH, Lamont SJ (2013) Genetics of disease resistance diseases of poultry. Application of Genetics and Genomics in Poultry Science 13: 70- 86.
- Levy S (1994) Balancing the drug resistance equation. Trends Microbiol 2(10): 341-342.
- Bierer BW, Eleazer TH, Roebuck DE (1965) Effect of feed and water deprivation on chickens, turkeys, and laboratory mammals. Poult Sci 44(3): 768-773.
- Altekruse SF, Stern NJ, Fields PI, Swerdlow DL (1999) Campylobacter jejuni -An emerging foodborne pathogen. Emerg Infect Dis 5(1): 28-35.
- Thormar H, Hilmarsson H, Bergsson G (2006) Stable concentrated emulsions of the 1-monoglyceride of capric acid (monocaprin) with microbicidal activities against the food-borne bacteria Campylobacterjejuni, Salmonella spp., and Escherichia coli. App Environ Microbiol 72(1): 522-526.
- Silva J, Leita D, Fernandes M, Mena C, Gibbs PA, et al. (2011) Campylobacter spp. as a foodborne pathogen: A Review. Front Microbiol 2: 1-200.
- Lu J, Ryu H, Domingo JWS, Griffith JF, Ashbolt N (2011) Molecular detection of campylobacter spp in California gull (larus californicus) excreta. Applied and Environmental Microbiology 77(14): 5034-5039.
- Bahrndorff S, Rangstrup CL, Nordentoft S, Hald B (2013) Foodborne disease prevention and broiler chickens with reduced Campylobacter infection. Emerg Infect Dis 19(3): 425-430.
- Venkatanarayanan K, Kollanoor JA, Michael P, Doyle (2014) Microbiological safety of foods In: Handbook of nutrition and food. In: Carolyn D, Berdanier, Johanna TD (Eds.), Taylor & Francis Group, LLC, USA.
- Sahin O, Morishita TY, Zhang Q (2002) Campylobacter colonization in poultry: Sources of infection and modes of transmission. Anim Health Res Rev 3(2): 95-105.
- Lee MD, Newell DG (2006) Campylobacter in poultry: filling an ecological niche. Avian Dis 50(1): 1-9.
- Humphrey T, O’Brien S, Madsen M (2007) Campylobacters as zoonotic pathogens: A food production perspective. Int J Food Microbiol 117(3): 237-57.
- Newell DG, Mughini GL, Kalupahana JA, Wagenaar JA (2017) Campylobacter epidemiology-sources and routes of transmission for human infection (Chapter 5). In: Klein G (Ed.), Campylobacter features, detection, and prevention of foodborne disease. Elsevier Academic San Diego, Cambridge, MA, USA, pp. 85-110.
- Suzuki H, Yamamoto S (2009) Campylobacter contamination in retail poultry meats and by-products in Japan: A literature survey. Food Control 20: 531-537.
- EFSA (European Food Safety Authority) (2010) Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008. Part A: Campylobacter and Salmonella prevalence estimates. AFSA J 8(3): 99.
- Adak GK, Meakins SM, Yip H, Lopman BA, O’Brien SJ (2005) Disease risks from foods, England and Wales,1996-2000. Emerg Infect Dis 11(3): 365- 372.
- Hall G, Kirk MD, Becker N, Gregory JE, Unicomb L, et al. (2005) Estimating foodborne gastroenteritis, Australia. Emerg Infect Dis 11(8): 1257-1264.
- Tam CC, O’Brien SJ, Tompkins DS, Bolton FJ, Berry L, et al. (2012) Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin Infect Dis 54(9): 1275-1286
- Tam CC, O’Brien SJ, Tompkins DS, Bolton FJ, Berry L, et al. (2012) Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin Infect Dis 54(9): 1275-1286
- Wooldridge KG, Ketley JM (1997) Campylobacter-host cell interactions. Trend Microbiol 5(3): 96-102.
- Zhang Q, Sahin O (2013) Campylo bacteriosis Diseases of Poultry. Willey Blackwell 13: 737-750.
- Ketley (1997) Pathogenesis of enteric infection by Campylobacter. Review article. Microbiology 143(Pt 1): 5-21.
- WHO (1997) The medical impact of the use of anti-microbials in food animals: Report and proceedings of a WHO meeting, Berlin, Germany, WHO/EMC/ZOO/97, pp. 13-17.
- Foley EG, Lee SW, Hartley NJ (1946) The effect of penicillin on staphylococci and streptococci commonly associated with bovine mastitis. J Food Technol 8: 129-33.
- Gillespie SH (2001) Antibiotic resistance in the absence of selective pressure. Int J Antimicrob Agents 17(3): 171-176.
- Normark BH, Normark S (2002) Evolution and spread of antibiotic resistance. J Intern Med 252(2): 91-106.
- Maisnier PS, Andersson DI (2004) Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res Microbiol 155(5): 360-369.
- Tortora G, Funke B, Case C (1995) Microbiology: An Introduction. In: Edwood R (Ed.), The Benjamin/Cummings Publishing Company, Colifornia, USA.
- Palácio SB, Garcia JE, Ferro Cavalcanti IM (2018) Microbial resistance due to the use of antimicrobials in livestock and agriculture dos Santos Medeiros SMFR. Approaches in Poultry Dairy and Veterinary Sciences 4(4): 1-5.
- Bachoual R, Ouabdesselam S, Mory F, Lascols C, Soussy CJ, et al. (2001) Single or double mutational alterations of gyrA associated with fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli. Microbl Drug Resist 7(3): 257-261.
- Gibreel A, Taylor DE (2006) Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J Antimicrob Chemother 58(2): 243-255.
- Alfredson DA, Korolik V (2007) Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Micorbiol Lett 277(2): 123-132.
- Hariharan H, Sharma S, Chikweto A, Matthew V, DeAllie C (2009) Antimicrobial drug resistance as determined by the E-test in Campylobacter jejuni, C. coli, and C. lari isolates from the ceca of broiler and layer chickens in Grenada. Comp Immun Microbiol Infect 3(1): 21- 28.
- Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM et al. (2009) Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol 4(2): 189-200.
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). (2013). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. The EFSA J 11(4): 3129.
- Blaser MJ, Engberg J (2008) Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I, Szymanski CM, Blaser MJ (Eds.), Campylobacter. ASM press, Washington DC, USA, pp. 99-121.
- Bester LA, Essack SY (2008) Prevalence of antibiotic resistance in Campylobacter isolates from commercial poultry suppliers in KwaZulu- Natal, South Africa. J Antimicrob Chemother 62(6): 1298-1300.
- Chen X, Naren GW, Wu CM, Wang Y, Dai L, et al. (2010) Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet Microbiol 144(1-2): 133-139.
- Fraqueza M, Martins A, Borges A, Fernandes MH, Fernandes MJ, et al. (2014) Antimicrobial resistance among Campylobacter spp. strains isolated from different poultry production systems at slaughterhouse level. Poult Sci 93(6): 1578-1586.
- Giannatale DE, Serafino DG, Zilli K, Alessiani A, Sacchini L, et al. (2014) Characterization of antimicrobial resistance patterns and detection of virulence genes in Campylobacter isolates in Italy. Sensors 4(2): 3308- 3322.
- Economou V, Zisides N, Gousia P, Petsios S, Sakkas H, et al. (2015) Prevalence and antimicrobial profile of Campylobacter isolates from free-range and conventional farming chicken meat during a 6-year survey. Food Control 56: 161-168.
- Boamah VE, Agyare C, Odoi H, Dalsgaard A (2016) Practices and factors influencing the use of antibiotics in selected poultry farms in Ghana. J Antimicrob Agents 2(2): 120.
- Beyene T, Kemal A, Jibat T, Tadese F, Ayana D, et al. (2015) Assessment on chemicals and drugs residue in dairy and poultry products in bishoftu and modjo. Central Ethiopia J Nutr Food Sc S13: S13-002.
- Nisar M, Ahmad MD, Mushtaq MH, Shehzad W, Hussain A, et al. (2017) Prevalence and antimicrobial resistance patterns of Campylobacter spp. isolated from retail meat in Lahore, Pakistan, Food Control 80: 327-332.
© 2018 Ahmed Marroki. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






