- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Q Fever (Coxiellosis) in Animals and Humans
Tewodros Alemneh1,3* and Melaku Ayelign2,3
1 Woreta Town Office of Agriculture and Environmental Protection, Ethiopia
2 Gondar City Administration, City Service Provision, Ethiopia
3 Faculty of Veterinary Medicine, University of Gondar, Ethiopia
*Corresponding author: Tewodros Alemneh, Faculty of Veterinary Medicine, University of Gondar, Ethiopia, Woreta Town Office of Agriculture and Environmental Protection, Woreta, South Gondar Zone, Ethiopia
Submission: December 17, 2018;Published: December 20, 2018

ISSN: 2576-9162 Volume5 Issue4
Abstract
Q fever is an acute and chronic zoonotic disease of highly public health importance worldwide. The disease is caused by an obligate gram-negative bacterium; Coxiella burnetii (C. burnetii). Coxiella burnetii belongs to the genus Coxiella of the gamma sub-division of Proteobaccteria along with the genera Legionella, Francisella, and Rickettsiella. Unlike the other members of Proteobaccteria, C. burnetii is highly resistant to adverse physical conditions and chemical agents, so it can survive for months and even years in the environment. Domestic animals such as cattle, sheep and goats act as the major reservoirs of C. burnetii which can infect a large variety of animals, humans, birds, and arthropods. Human infection results from inhalation of contaminated aerosols, consumption of contaminated unpasteurized dairy products, direct contact with contaminated milk, urine, feces, or semen of infected animals, and tick bites. Clinical presentation is non-specific and highly variable ranging from asymptomatic infection or self-limiting febrile illness to a typical rapidly progressive pneumonia and/or hepatitis. In animals, Q fever is frequently asymptomatic. Sheep and goats may exhibit abortion, stillbirth, pre-mature delivery, and delivery of weak offspring while cattle and camel may develop infertility, metritis, and mastitis. Diagnosis and isolation of C. burnetii are mainly focused on molecular tests (PCR), and serological tests such as Complement Fixation Test (CFT), Enzyme-Linked Immunosorbent Assay (ELISA) and Indirect Fluorescence Assay (IFA). Doxycycline and Oxytetracycline are the drug of choice for the treatment of Q fever infection. Due to its wide range of reservoir hosts and severity of infection both in animals and humans, emphasis should be given in the control and prevention of this zoonotic disease.
Keywords: Q fever; Coxiella burnetii; Zoonosis; Animals; Humans
Introduction
Q fever is a zoonotic bacterial infection caused by Coxiella burnetii, an obligate intracellular parasite, classified within the family Rickettsiaccae. Q fever (Q for “query”) was first used in 1937 to describe a mysterious febrile illness of packing house workers in Brisbane, Australia. The causative agent was isolated from infected workers and later was identified as Rickettsiae. Almost simultaneously the same organism was identified wood tick collected Montana. Cox, an American, and Burnet, an Australian, where honored for their early work with this organism, hence the name “Coxiella burnetii”. Along with trench fever and epidemic typhus, Q fever caused epidemics in the armies fighting in Europe in World War II [1].
Although the clinical disease is limited to humans, a wide range of domestic and free living ungulates are naturally infected. It affects a very wide range of hosts in cattle, buffalos, camels, sheep, goats, pigs, dogs, cats and wild and domestic birds which can be infected without clinical sign. In pregnant, the pathogen enters the uterus which leads to abortion. Late abortion is the commonest expression in ruminants’ particularly in sheep and goats, either sporadically or in sudden outbreaks [2].
Zoonosis of Q fever is associated primarily with parturient ruminants, although domestic animals such as cats and a variety of wild animals have also been involved with human infection. It occurs more frequently in persons of occupational contact with high risk species. It has highly variable clinical presentation in humans, ranging from self-limiting influenza like illness to pneumonia, hepatitis, and endocarditis. It is highly infectious; a single organism of Coxiella burnetii can reportedly cause infection through the aerosol route in humans [3].
Urine, faeces and birth material of infected animals are sources for C. burnetii contamination of the environment. The shedding of the bacteria is the highest during birth or abortion [4,5]. In addition, direct contact with contaminated products in establishments processing either the infected animals or their by-products (like straw, hides or wool) may also cause infection. To a lesser extent, infection may occur via the gastro-intestinal route (raw dairy products as milk and meat) and human-to-human transmission (through blood transfusion or sexual transmission) [5,6].
Unlike other Rickettsias, these organisms can survive long periods outside cells and can be transmitted through air as well as by ticks. They can survive six months in dried blood, seven to nine months in wool, and over two years in water or skim milk [7]. Their broad range of hosts and complicated vector born transmission cycle have lead them ubiquitous organisms [8] having worldwide distribution and complex ecology and epidemiology [1]. Q fever is considered as a potential agent for bioterrorism; due to its stability in the environment, the ease with which it can transmit by aerosol and very low infectious dose [9].
The ubiquitous distribution of these organisms in the natural environment, the complexity of their epidemiology, and their human health consequences acquired by direct or indirect contact with animals’, predicate the need for continued attention and control. Therefore, continued attention and detailed knowledge of the organism is vital for understanding of Q fever and to undertake prevention and control strategies against the disease. Hence, the objective of this work is to review Q fever both in animals and humans.
Background and etiology
It was first described by Edward Holbrook Derrick [10] in abattoir workers in Brisbane, Queensland, Australia. The “Q” stands for “query” and was applied at a time when the causative agent was unknown; it was chosen over suggestions of “abattoir fever” and “Queensland rickettsial fever,” to avoid directing negative connotations at either the cattle industry or the state of Queensland [11]. The pathogen of Q fever was discovered in 1937, when Frank Macfarlane Burnet and Mavis Freeman isolated the bacterium from one of Derrick’s patients [12]. It was originally identified as a species of Rickettsia. H.R. Cox and Gordon Davis isolated it from ticks in Montana, USA in 1938 [13]. Coxiella burnetii is no longer regarded as closely related to Rickettsiae, but as similar to Legionella and Francisella, and is a Proteobacterium [14].
Coxiella burnetii is a gram negative, non-motile intracellular bacterium [15], pleomorphic, ovoid to rod-like in shape that belongs to the gamma sub-division of the Proteobacteria [8].
Molecular taxonomy: Critical to the current understanding of the epidemiology and epizootiology of Q fever are the development in biotechnology that allows the characterization of isolates at the molecular level. Advance in the application of recombinant DNA technology to the study of Coxiella burnetii has led to the discovery of significant genetic difference and similarities among the microorganism isolated from arthropods and numerous mammalian sources. The genetic differences and similarities analyzed by genomic restriction fragment length polymorphism (RFLP) using Bamh1 restriction endonuclease digests and denaturing polyacrylamide gel electrophoresis reveals six genomic groups. It is the primary basis for classification scheme that includes plasmid type, genomic hybridization, Lipopolysaccharides, microheterogeneity and antigenic variation, physical mapping genome, disease association and animal virulence [8].
Genomic group contains reference Coxiella burnetii strain that were isolated from infected human end/or animals [16]. Strain in genomic group 1, 2 and 3 have been isolated from tick, human blood (acute Q fever), from milk of persistently infected dairy cattle, and/or aborted fetal tissue. Strains in genomic group 4 and 5 have been isolated from the heart of humans with chronic Q fever and/ or aborted tissue of animals. Strains in genomic group 6 have been isolated only from rodents; these strains are of unknown virulent for humans and animals [8].
Phase variation: Coxiella burnetii has two antigenic surface phases; phase I and phase II, which vary in their pathogenic and immunogenic properties and undergo antigenic phase variation [9] due no Lipopolysaccharide truncation [17] when serially passaged in embryonated eggs [9] or cell culture [17]. The phase I form is extremely infectious and exists in human and other animals. Passaging can also result in a shift to form spore which allows the organism to survive in harsh environment. Indeed, it can survive for greater than 40 months in skim milk at room temperature and is readily recovered from soil up to one month after contamination [17]. It has high level of resistance to chemical and physical agents. High level of resistance to heat has been attributed to the formation of endospore [18].
Cultural and staining characteristics: Coxiella burnetii does not grow in artificial media, but can be cultured in embryonated eggs, primary cells, several cell lines including Vero cells and a number of macrophage-like cell lines [9]. However, the procedure is restricted to specialized laboratories because of attendant zoonotic risk. Slain red with modified Ziehl Nelson and Machiavello procedures, and purple with Giemsa stain [19]. They are gram negative, non-motile and varies in shape from pleomorphic to ovoid even to rod like [8]. On adaptation to laboratory culture, Coxiella burnetii undergoes modification of antigenicity and virulence from phase I (analogous to smooth gram negative bacteria) found in animals to phase II (rough from). However, this variation of antigenicity has relevance for vaccine formation and serological diagnosis [19].
Occurrence: Q fever may occur in sporadic as well as epidemic forms. It may be emerging disease, probably related to climate changes [20]. Q fever is an endemic zoonosis with worldwide distribution, except Antarctica and New Zealand [21]. The patterns of transmission makes the epidemiology of C. burnetii complex, there are two major ways of spread involving wild animals and their ectoparasites, mainly ticks; and the other involving domestic ruminants, independent to wild animal cycle [3]. The broad host range and complicated vector born transmission cycle have led to the ubiquity of Coxiella burnetii among arthropods annelid, poiklotherms, and the homoeothermic reservoirs in disparate habitat. Although Q fever is ubiquitous, they occur more frequently in area where domestic animals are prevalent [8].
Host and susceptibility: Coxiella burnetii is a well-established infectious agent that has reached a state of balanced pathogenicity in a plethora of host. Human are unnatural and usually dead-end-host. The microorganism generally maintained in a less pathogenic form in separate cycle existing independently among wild mammals and their haematophagus arthropods, principally tick and in domestic animals [8].
The reservoir hosts vary depending on the geographic location, and include domestic and wild animals, and their ectoparasites [21]. The primarily reservoir hosts for C. burnetii are ticks, both Ixodid and Argasid, which facilitate wild life cycle in rodents, large animals, and birds [8]. Bird may serve as reservoirs; there was one outbreak associated with exposure to aerosol of pigeon faces and bite from tick feeding on these birds. Wildlife and farm animal species may be the reservoir hosts for domestic pets [21].
Source of infection and transmission: In humans, infection is primarily by inhalation. Sources of infection include such diverse materials contaminated by infected urine, faeces or birth products of animals as indicated in Figure 1. Potential sources for human infection are substantial; ovine manure used as garden fertilizer has been incriminated as a source. Sheep have traditionally been incriminated as the major reservoir of infection for human, but the trend for urban population to locate in close proximity to large dairy herds suggests that cattle could become an increasingly significant reservoir. Several incident of infection in human have been exposure to parturient sheep and goats. Immuno compromised people are at high risk for disease. There is a concern that the prevalence of infection in farm animals is increasing and spread geographically and that there is a subsequent greater risk for bioterrorism because of its survival in the environment, the ease with which it can be transmitted by aerosol and wind-borne means and the very low infectious dose [9].
Figure 1:Sources of Coxiella burnetii for human infection [8]

Infection of non-pregnant animals is clinically silent and is followed by latent infection until pregnancy when there is recrudescence with infection in the intestine, uterus, placenta and udder, and excretion from this site at parturition. The organism is present in high concentration in the placenta and fetal fluids, and subsequent vaginal fluids. It is also excreted in urine, and through milk [9]. Cattle and small ruminants can shade the organism through faeces, sperm and reproductive discharge. Parturient cats and dogs have also been implicated as source of human infection [22].
Transmission is by direct contact and through inhalation. There is significant contamination of the environment of infected animals at the time of parturition and this is probably a critical period for transmission of the disease within herds and flocks [9]. Coxiella burnetii is transmitted from various reservoir hosts to human through direct contact as well as air born, vector born, and through contaminated vehicle. In ticks, there is transovarian (transmission of Coxiella burnetii from adult tick to egg) and transstadial (transmission of Coxiella burnetii from larvae to nymph, and adult) [8]. The organism is present in the semen of seropositive bulls and venereal transmission is suspected [9].
Many arthropods including cockroaches, beetles, flies, fleas, bugs, lice, mite and ticks are naturally or experimentally infected; human rarely acquired the disease by bites of arthropods [23]. Most human cases of Q fever can be traced to exposure to infected sheep, cattle and goats either directly or through unpasteurized milk [9,21,22].
Pathogenesis and pathogenicity
After Coxiella burnetii enter to the host and gain access to vascular endothelium and respiratory and renal epithelium, it multiplies in phagosomes; due to an enzyme system adapted low PH (5.0). It causes necrosis and hemorrhage of many other organs including the liver, central nervous system and mononuclear phagocytic system. In animals the pattern may be similar, although mildly affected animals may have latent infection. Latent infection can persist particularly in the locating mammary gland and the pregnant uterus but become reactivated during parturition. Abortion (sporadically) may result from placentitis or the delivery may be normal and produce viable young. Immune complex pneumonia can develop in many organs. Chronically infected animals may continue to shade organisms in their urine and faeces [21].
Coxiella burnetii has pathogenicity that is resistant to physical and chemical influences and can survive in the environment and soil for several months. It can resist common chemical disinfectants, but is susceptible to sodium hydrochlorite (NaHCl), Lysol solution and formalin fumigation provided a highly humidity is maintained. After recovery, the virulent organism can persist in mononuclear phagocytes and people can be latently infected with Coxiella burnetii for extended periods [21].
Clinical manifestation
In animals, the infection is most often unapparent and can occur at any age. The commonest expression of disease is late abortion in ruminant animals, particularly in sheep and goats, either sporadically or in sudden outbreaks [9] with subsequent excretion of large number of bacteria. Such excretion is also possible in unapparently normal deliveries [15]. Unlike most other rickettsial diseases, skin rash is not a part of clinical syndrome [1]. Splenomegaly is usually the only finding in acutely infected dogs [21]. In pregnant animal, it may lead premature abortion or still birth. Animals with chronic infection manifest myocarditis, pericarditis, thrombosis in various organs, bone marrow granuloma and necrosis, and chorioamionitis [15].
In humans, the disease appears in acute and chronic forms. Acute forms are generalized one, resembling influenza and the incubation period ranges from 2-4 weeks. There is sudden fever occasionally up to 40°C and other symptoms include chills, sweat, vomiting and diarrhea which occur in 5-20% of patients. Coughing, pneumonia, and neurologic manifestations are uncommon in acute Q fever; however, in one outbreak in the west midlands, United Kingdom, 23% of 102 patients had neurologic signs and symptoms as the major manifestation. Symptomatic cases in children result in Spectrum of diseases similar to that in adults, although only few eases of Q fever endocarditis of children have been reported [17]. In chronic case endocarditis usuallyy occurs in patients with pre-existing valvulopathy or those who are immunosuppressed [15]. Patients with chronic Q fever have hepatomegaly and lymphadenophy [24]. Q fever endocarditis follows an unapparent or sub-clinical infection and mainly affects the mitral and aortic valves. The usual symptoms are moderate fever, night-sweet, anorexia and variable cardiac murmurs [15].
Diagnosis
Because of laboratory acquired infection caused by C. burnetii, cultivation of the organism has been discouraged. However, the use of a shell vial assay with human lung fibroblast to isolate the organism from buffy coat and biopsy specimens has not resulted in any laboratory acquired infection [25]. Smear from placental tissue and urine discharge stained by the Modified Ziehl Neelson (MIN) method reveal small clumps of road coco-bacillary bodies [26]. Rickettsiae can be identified in placental impression smear stained with modified Kostor’s stain, stump’s MZN as pleomorphic acid fast cocoid or filamentous organisms in trophoblast or extra extracellularly [27]. Isolation must be made in cell culture of macrophage or fibroblast lines, embryonated eggs or laboratory rodents, but cell dissociated to phase II because phase I is highly infectious. Phase I antigens are isolates from organisms taken directly from animals or their patients. This natural phase is highly infectious, contain large amount of lipopolysaccharides (LPs), and form smooth colonies in culture. Phase II antigens are round in organisms that have been passed serially in embryonated eggs, have a truncated, and lack some cell surface antigens [21].
Serology is the most convenient and commonly used diagnostic tool. Three serological techniques are available: Indirect Fluorescence Antibody (IFA), Complement Fixation Test (CFT) and Enzyme Linked Immuno Sorbent Assay (ELISA). IFA is considered the reference method for both acute and chronic Q fever that is highly specific and sensitive, and is recommended for its reliability, cost effectiveness, and ease of performance ([25]). Polymerase Chain Reaction (PCR) or immuno histochemical methods can be used for detection of Coxiella burnetii in tissue culture or tissue specimens derived from patients [22].
In animals, it should be differentiated from diseases causing abortion [9]. In human, C. burnetii should be differentiated clinically from viral, mycoplasma, and bacterial pneumonia; viral hepatitis; tuberculosis; and from other animal borne diseases [24]. Furthermore, salmonellosis, leptospirosis, and less sever diseases due to Rickettsia should be included. In tropical areas, malaria should also be suspected in case of humans [15].
Treatment
Doxycycline is the most effective drug against C. burnetii, in most reports [28-30]. However, strains with acquired resistance to Doxycycline have been described and represent a worrisome situation. The first resistant strain was isolated from a patient who died from C. burnetii endocarditis. In the same study, Rolain et al. [31] found a correlation between the ratio of serum concentration to MIC for doxycycline and the rate of decline of anti-C. burnetii antibody titers in patients with C. burnetii endocarditis. For 16 C. burnetii strains isolated from cardiac valves removed from endocarditis patients, a ratio of serum concentration to MIC of >1 correlated with a rapid decline in specific antibody titers. A ratio between 0.5 and 1 was associated with a slower reduction in antibody titers. The only patient who died from endocarditis had a ratio of < 0.5. The whole genome of the C. burnetii strain infecting that patient was determined, but no specific sequence could be correlated with doxycycline resistance [32]. Since then, two other isolates have been found to be resistant to doxycycline, including one goat isolate and another human isolate from a patient with acute Q fever [33].
In early studies, the fluoroquinolones were found to be one of the most effective agents in eliminating C. burnetii from L929 cells [34]. For that reason, in 1989 it was proposed to combine doxycycline with a fluoroquinolone to treat persistent forms of C. burnetii infection (30). Fluoroquinolones are also recommended for treatment of acute meningitis caused by C. burnetii because of the good cerebrospinal fluid penetration of these drugs [35]. Pefloxacin- or ciprofloxacin-resistant strains of C. burnetii have been selected in vitro by Spyridaki et al. [29] and Musso et al. [36] with MICs up to 64mg/liter. These authors’ identified point mutations in the gyrA gene that could allow PCR- restriction fragment length polymorphism (PCR-RFLP) detection of these resistant strains [32]. However, to date, clinical isolates of C. burnetii remain susceptible to Levofloxacin, Moxifloxacin, and to a lesser extent Ciprofloxacin [37].
Erythromycin was proposed as an empirical treatment for C. burnetii pneumonia. However, in 1991, Raoult et al. [38] found that 6 of 13 clinical isolates of C. burnetii were resistant to this antibiotic, and such resistance was more recently observed in 6 isolates from Cayenne, French Guiana [39,40]. Conversely, clarithromycin was found to be active, with MICs between 2 and 4mg/liter [41,42]. For Azithromycin, higher MICs, up to 8mg/liter, have been observed [38,40]. Telithromycin was considered active against C. burnetii, with MICs between 0.5 and 2mg/liter for 13 clinical isolates [33]. However, Eldin et al. [37] recently isolated a strain from French Guiana which was resistant to this antibiotic.
No resistance to Sulfamethoxazole-trimethoprim has been reported to date, suggesting that this agent is useful during pregnancy. Anecdotal reports observed susceptibility to Tigecycline and Linezolid and proposed them as alternative agents [42,43]. Unsworth et al. [44] recently reported susceptibility of C. burnetii to antimicrobial peptides. Other non-antibiotic agents have been reported to display in vitro activity against C. burnetii [37].
Although C. burnetii is susceptible to a number of antibiotics, as discussed earlier, Oxytetracycline is the preferred choice [19]. Treatment with tetracycline in four divided doses can suppress symptoms and shorten the clinical course, but does not always eradicate the infection [24]. Treatment in animal is not routine procedure, but may assist in control of clinical problem in which the disease is implicated [19]. The major challenge for successful therapy is that the phagosomes, Coxiella burnetii persist, which are highly acidic where most antimicrobial agents are not affective. Alkalizing cell by using Chloroquine has been shown to improve clinical efficacy of Chloramphenicol, Enrofloacin and Trimethoprim sulfa [22].
Control and prevention
Measures to identify and decontaminate infected areas and to vaccinate domestic animal population are difficult, expensive, and impractical [1]. Experimental develop most for use in animal have incorporated inactivated whole cell or extracts of Coxiella burnetii. Usually organism in phase II are used because they are safer to handle during bulk culture, but some vaccines have been based on phase I organisms, which antigenically are more closely related to those occurring in natural [19]. Extended studies by Biberstein [45] employed a phase I formalin inactivated Q fever vaccine which provide effective in the immunization of dairy cattle. Administration of formalin inactivated phase I Coxiella burnetii vaccine in naturally infected ewes and cows eliminate shedding of the organism in milk. Experimentally, dogs have been vaccinated with formalin inactivated phase I and phase II antigens and have developed humeral and cell-mediated immunity in response to Coxiella burnetii [21].
Aborted animals should be isolated for 3 weeks and aborted and placental contaminated material should be burned. Ideally, manure should be composted for 6 months before application to fields. Feed areas should be raised to keep them free from contamination with faeces and urine. Milk and milk products should be pasteurized [9].
Segregation of parturient ruminants and careful disposal of placenta and aborted fetuses are essential after a diagnosis have been confirmed [26]. Based on detection of the infection in livestock, it is necessary to reduce contact with infected animals or dust contaminated by them. Special care should be taken when working with animal tissue, and effective pasteurization of milk [24]. People at high risk of contracting to Coxiella burnetii, such as veterinarians, shepherds, abattoir workers, and laboratory personnel should receive vaccine [46]. Protective gloves and garments, including masks and protective eye wear, should be worn when performing procedure involving the genital tissue or secretion of patient or aborted animals [21]. Veterinarian dealing with herds that provide row milk should insure that this herd sero-negative for Coxiella burnetii [9].
Prevalence and Zoonotic Implication
Table 1:Studies on prevalence and disease infection of Coxiella burnetii in humans and animals in Northern and Western Regions of Africa.
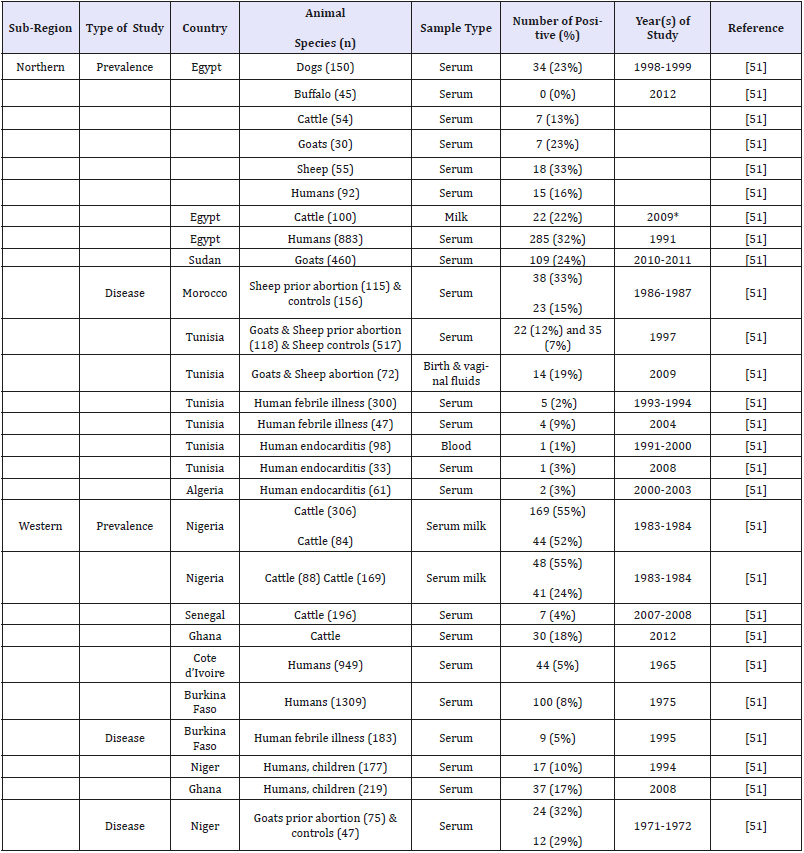
Table 2:Studies on prevalence and disease infection of Coxiella burnetii in humans and animals in the middle, Southern and Eastern Regions of Africa.
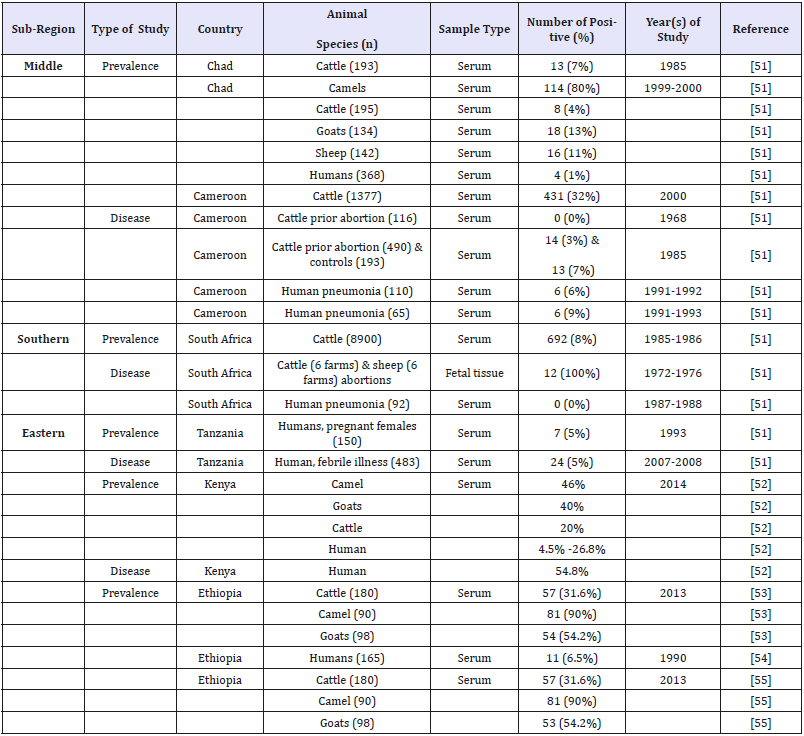
With occasional exceptions, animal coxiellosis has not been regarded as an important animal health problem. However, with increasing attention now being paid to zoonosis, the role of livestock as the reservoir of Coxiella burnetii will come under closer scrutiny. By no means, all cases of human Q fever are traceable to livestock and there is growing evidence that other sources, such as parturient cats, are implicated in some instance [19]. Environment exposures that account for outbreaks of Q fever are numerous. Occupational exposures in abattoirs and research facilities still account for the majority of reported cases in United States. However, worldwide, Q fever cases are reported more frequently and massive outbreaks have been documented. Estimate of Q fever infection rate in Netherland vary from 45% in endemic areas to 75.9% in humans of highly hazardous occupations [8]. Most community outbreaks have been associated with the lambing / calving period of goats or sheep and have been temporally linked to the lambing season in high density rearing areas [47,48]. The movement of domestic ruminants and their products (either for consumption or via fomites) has been linked to propagated human outbreaks in Bulgaria, France, Switzerland, Britain and The Netherlands [49,50]. Two outbreaks in Britain and Switzerland demonstrated that residents living along roads through which vehicles of sheep travelled could become infected as a result of exposure to contaminated straw or dust [50]. Several outbreaks of Q fever were registered worldwide, in which, Asian, North American and European countries took part too. According to the report of Clark and Magalhães [50], the United Kingdom was affected in 1989, France in 1998, Germany in 2005, and the Netherlands in 2007. The disease is also common in Africa, and its distribution is shown in Table 1 & 2 [51-55].
Conclusion
Q fever is a zoonotic bacterial disease of worldwide distribution. All people in the world should concern because of its importance causing abortion, still birth and birth of poorly viable newborns especially in sheep. However, successive pregnancies are normal, but there is recrudescence of infection and excretion of the organism in these pregnancies, especially in the one immediately following. Coxiella burnetii is highly infectious to human; even a single organism can cause infection and clinical disease is limited largely to humans; therefore, even greater concern should be paid for its importance as a zoonosis. Because of its stability in the environment, the ease with which it can transmitted by aerosol, and very low infectious dose and variable clinical presentations even it can be considered as a potential agent for bioterrorism.
Therefore, the following points have been forwarded as recommendations: animal producers should be aware concerning the economic effect and public health significance of coxiellosis. Hygienic measures such as disinfection of utensils, destruction of placenta, contaminated bedding and dung should be made. In countries like Ethiopia, where the epidemiology of the disease is not well known, people at high risk should be concerned for Q fever while they have direct or indirect contact with animals and their secretions.
Acknowledgement
Authors highly intended to acknowledge Dr. Sefinew Alemu for his special advice sharing experiences and devotion of time in correcting this work. Kind cooperation of the staff of Faculty of Veterinary Medicine, University of Gondar, was also worthwhile for the success of this review.
Conflicting of Interest
Authors declare that no any conflicting of interests in the publication of this work.
References
- Joklik KW, Willett, Ames, Willett PH, Amos BD, Wilfert (1992) Zinsser Microbiology. (20th edn), Appleton and Mange, New York, USA, pp. 7 l 3-714.
- Seifert HSH (I992) Coxiellosis (Q fever): Tropical animal health. Kluwer Academic Publishes, London, UK. pp. 2004-4007.
- Brooks FG, Butel SJ, Morse AS (2004) Medical Microbiology. (23rd edn), Mc Grow Hill. Boston, USA, p. 35.
- Berri M, Rousset E, Champion JL, Russo P, Rodolakis A (2007) Goats may experience reproductive failures and shed Coxiella burnetii at two successive parturitions after a Q fever infection. Res Vet Sci 83(1): 47-52.
- Meerburg BG, Reusken CBEM (2011) The role of wild rodents in spread and transmission of Coxiella burnetii needs further elucidation. Wildlife Research 38(7): 617-625.
- Angelakis E, Raoult D (2010) Q fever. Veterinary Microbiology 140 (3-4): 297-309.
- Creagor GJ, Black GJ, Davision EV, Mathai CW (I990) Microbiology principles and applications. Pronticc Hall, Englewood cliffs, USA, p. 576.
- Beran WG, Steele HJ (I994) Hand book of Zoonosis. (2nd edn), CRS press, Baca Raton, USA, pp. 430-431.
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2007) Veterinary medicine, a text book of the diseases of cattle. Sheep, Pigs, Goats and Horses. (10th edn), Saunders publisher, Edinburgh, UK, pp. 1468-4469.
- Derrick EH (1983) Q’ fever, a new fever entity: Clinical features, diagnosis and laboratory investigation. Rev Infect Dis 5(4): 790-800.
- Marrie TJ, Ed (1990) Historical aspects of Q fever. In: Q Fever, The Disease, CRC Press, Boca Raton, Fla, USA.
- Burnet FM, Freeman M (1937) Experimental studies on the virus of Q fever. Medical Journal of Australia 2(8): 299-305.
- Davis GE, Cox HR (1938) A filter-passing infectious agent isolated from ticks. I. Isolation from Dermacentor andersonii, reactions in animals, and filtration. Public Health Reports 53(52): 2259-2282.
- Honarmand H (2012) Q Fever: An old but still a poorly understood disease. Interdiscip Perspect Infect Dis 2012: 131932.
- Hartmut K, Albert W, Burkhard E, Hennery DL, Hans GS, et al. (2003) Infectious disease transmissible from animal to humans. (3rd edn), ASM press, Washington DC, USA. pp. 229-230.
- Cupte S (2002) The short text book of medical microbiology. (8th edn), Mc Grow Hill, New Delhi, India, pp. 267-269.
- Kasper LD, Fouci SA, Longo LD, Braunwald E, Hauser LS, et al. (2005) Harrison’s principles of internal medicine. (16th edn), Medical publishing division, New York, USA, pp. 107-108.
- Varnam AH, Evans MG (I991) Food borne pathogens. Manson, London, UK. pp. 357.
- Houlard, Jimmy (1986) Current veterinary therapy. (3rd edn), W. B. Saunders, London, UK, pp. 622-623.
- Gebremedhin Y, Shallom M (2018) Review on Q fever in small ruminants and its public health importance. Biomed J Sci&Tech Res 9(1): 1-10.
- Green EC (2006) Infectious diseases of the dogs and cats. (3rd edn), Saunders, Athens, Greece, pp. 242-245.
- Hrish CD, Machachlan JM, Walker RL (2004) Veterinary microbiology. (2nd edn), Black well Science, USA. pp. 251-252.
- Chakraborty (1995) A text book of microbiology. New Central book agency, India, p. 235.
- Tierney FJ, Gillespie HJ, Scott WF, Barlough EJ (I998) Hagan and Bruner’s microbiology and infectious disease of domestic animals. (8th edn), Comstock Publishing Association, London, UK, pp. 341-342.
- Forbes AB, Daniel FS, Alices SW (2002) Diagnostic microbiology. (11th edn), An affiliate of Elsevier, Mosby, USA, p. 583.
- Quinn JP, Mauarkey KB, Carter, EM, Donnely CWJ, Leonard CF (2002) Veterinary microbiology and microbial disease. (1st edn), Black well science Department of Microbiology and Parasitology, Great Britain, pp. 21l-2120.
- Smith PB (2002) Large animal internal medicine. (3rd edn), St. Louis, Mosby, USA, p. 1317.
- Lever MS, Bewley KR, Dowsett B, Lloyd G (2004) In vitro susceptibility of Coxiella burnetii to azithromycin, doxycycline, ciprofloxacin and a range of newer fluoroquinolones. Int J Antimicrob Agents 24(2): 194-196.
- Spyridaki I, Psaroulaki A, Vranakis I, Tselentis Y, Gikas A (2009) Bacteriostatic and bactericidal activities of tigecycline against Coxiella burnetii and comparison with those of six other antibiotics. Antimicrob Agents Chemother 53: 2690-2692.
- Eldin C, Perreal C, Mahamat A, Djossou F, Edouard S, et al. (2015) Antibiotic susceptibility determination for six strains of Coxiella burnetii MST 17 from Cayenne, French Guiana. Int J Antimicrob Agents 46(5): 600-602
- Rolain JM, Boulos A, Mallet MN, Raoult D (2005a) Correlation between ratio of serum doxycycline concentration to MIC and rapid decline of antibody levels during treatment of Q fever endocarditis. Antimicrob Agents Chemother 49(7): 2673-2676.
- Rouli L, Rolain JM, El Filali A, Robert C, Raoult D (2012) Genome sequence of Coxiella burnetii 109, a doxycycline-resistant clinical isolate. J Bacteriol 194(24): 6939.
- Rolain JM, Lambert F, Raoult D (2005b) Activity of telithromycin against thirteen new isolates of C. burnetii including three resistant to doxycycline. Ann N Y Acad Sci 1063: 252-256.
- Jabarit Aldighieri N, Torres H, Raoult D (1992) Susceptibility of Rickettsia conorii, R. rickettsii, and Coxiella burnetii to PD 127,391, PD 131,628, pefloxacin, ofloxacin, and ciprofloxacin. Antimicrob Agents Chemother 36(11): 2529-2532.
- Drancourt M, Gallais H, Raoult D, Estrangin E, Mallet MN, et al. (1988) Ofloxacin penetration into cerebrospinal fluid. J Antimicrob Chemother 22(2): 263-265.
- Musso D, Drancourt M, Osscini S, Raoult D (1996) Sequence of quinolone resistance-determining region of gyrA gene for clinical isolates and for an in vitro-selected quinolone-resistant strain of Coxiella burnetii. Antimicrob Agents Chemother 40(4): 870-873.
- Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, et al. (2017) From Q fever to Coxiella burnetii infection: A paradigm change. Clin Microbiol Rev 30(1): 115-190.
- Raoult D, Torres H, Drancourt M (1991) Shellvial assay: Evaluation of a new technique for determining antibiotic susceptibility, tested in 13 isolates of Coxiella burnetii. Antimicrob Agents Chemother 35(10): 2070-2077.
- Sandoz KM, Sturdevant DE, Hansen B, Heinzen RA (2014) Developmental transitions of Coxiella burnetii grown in axenic media. J Microbiol Methods 96: 104-110.
- Boulos A, Rolain JM, Maurin M, Raoult D (2004) Measurement of the antibiotic susceptibility of Coxiella burnetii using real time PCR. Int J Antimicrob Agents 23(2): 169-174.
- Enright JB, Sadler WW, Thomas RC (1957) Pasteurization of milk containing the organism of Q fever. Am J Public Health Nations Health 47(6): 695-700.
- Huebner RJ, Hottle GA, Robinson EB (1948) Action of streptomycin in experimental infection with Q fever. Public Health Rep 63(12): 357-362.
- Gikas A, Spyridaki I, Scoulica E, Psaroulaki A, Tselentis Y (2001) In vitro susceptibility of Coxiella burnetii to linezolid in comparison with its susceptibilities to quinolones, doxycycline, and clarithromycin. Antimi crob Agents Chemother 45(11): 3276-3278.
- Unsworth NB, Dawson RM, Wade JD, Liu CQ (2014) Susceptibility of intracellular Coxiella burnetii to antimicrobial peptides in mouse fibroblast cells. Protein Pept Lett 21(2): 115-123.
- Biberstein EL, Crenshaw GL, Behymer DE, Franti CE, Bushnell RB, et al. (1974) Dermal reactions and antibody responses in dairy cows and laboratory animals vaccinated with Coxiella burnetii. Cornell Vet 64(3): 387-406.
- Levinson W (2004) Medical microbiology and immunology. (8rd edn), Medical publishing division, New York, USA. pp. 117-118.
- Orr H, Christensen H, Smyth B, Dance D, Carrington D, et al. (2006) Case-control study for risk factors for Q fever in Southwest England and Northern Ireland. Eur Surveill 11(10): 13-14.
- Porten K, Rissland J, Tigges A, Broll S, Hopp W, et al. (2006) A superspreading ewe infects hundreds with Q fever at a farmers’ market in Germany. BMC Infect Dis 6: 147.
- Panaiotov S, Ciccozzi M, Brankova N, Levterova V, Mitova Tiholova M, et al. (2009) An outbreak of Q fever in Bulgaria. Ann Ist Super Sanita 45(1): 83-86.
- Clark NJ, Magalhães RJS (2018) Airborne geographical dispersal of Q fever from livestock holdings to human communities: A systematic review and critical appraisal of evidence. BMC Infect Dis 18(1): 218.
- Vanderburg S, Rubach MP, Halliday JEB, Cleaveland S, Reddy EA, et al. (2014) Epidemiology of Coxiella burnetii Infection in Africa: A OneHealth Systematic Review. PLoS Negl Trop Dis 8(4): e2787.
- Njeru J, Henning K, Pletz MW, Heller R, Neubauer H (2016) Q fever is an old and neglected zoonotic disease in Kenya: A systematic review. BMC Public Health 16: 297.
- Gumi B, Firdessa R, Yamuah L, Sori T, Tolosa T, et al. (2013) Seroprevalence of Brucellosis and Q-Fever in Southeast Ethiopian Pastoral Livestock. J Vet Sci Med Diagn 2(1).
- Abebe A (1990) Prevalence of Q fever infection in the Addis Ababa abattoir. Ethiop Med J 28(3): 119-122.
- Gumi B (2013) Mycobacteria and zoonoses among pastoralists and their livestock in south-East Ethiopia. Doctoral Thesis, University of Basel, Faculty of Science, Switzerland, pp. 1-142.
© 2018 Tewodros Alemneh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






