- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Potential of Spray Dried Animal Plasma as an Alternative In-Feed Growth Promoter in Poultry Production
Lea S Young and Yewande O Fasina*
Department of Animal Sciences, North Carolina Agricultural and Technical State University, Greensboro, NC 27411 USA
*Corresponding author: Yewande O Fasina, Department of Animal Sciences, North Carolina Agricultural and Technical State University, Greensboro, NC 27411 USA, Tel: 336-285-4805; Emailyfasina@ncat.edu
Submission: November 25, 2018;Published: November 29, 2018

ISSN: 2576-9162 Volume5 Issue3
Abstract
The presence of antibiotic-resistant bacteria in the gastrointestinal tracts of poultry and livestock have continued to increase governmental and consumer pressure on the food animal industry to halt the use of antimicrobials in animal feed. Research efforts in this regard have continued to focus on identifying alternative biogenics and nutraceuticals. This article reviewed scientific literature on the potential of spray-dried animal plasma as a biogenic alternative to antibiotic growth promoters in animal feed, particularly live poultry. Topics discussed herein include the documented beneficial effects of spray-dried animal plasma as a growth-promoter in various animal species, and its consequent application(s) in Poultry Production and Health.
Keywords: Spray-dried animal plasma; Non-antibiotic growth promoter; Poultry production
Introduction
Annually, about 48 million people are reported to get sick with foodborne illness, and 128,000 are hospitalized [1]. The top five microorganisms that have been implicated in causing foodborne illnesses are Norovirus, Salmonella spp., Clostridium perfringens, Campylobacter spp., and Staphylococcus aureus [2,3]. The foodborne illnesses caused by Salmonella spp., Clostridium perfringens, and Campylobacter spp., are closely linked to poultry, and four out of the top five microorganisms are caused by bacteria [2,3]. About two million people are infected with antibiotic-resistant bacteria, with at least 23,000 people succumbing to their infection [4].
The United States poultry industry is globally the largest producer and second largest exporter of poultry meat, and a major egg producer [5]. Poultry is one of the major meat types consumed in America, and this has prompted farms to increase production. There has been a shift from small poultry farms to integrated intensive rearing systems in which poultry companies are structured to produce millions of chickens each week. In 2014, the U.S. poultry industry produced 8.54 billion broilers, 99.8 billion eggs, and 238 million turkeys [6]. Such intensive rearing systems increase the opportunity for diseases to spread rapidly in poultry flocks. Strategies that have been typically used by the poultry industry to control diseases include the implementation of vaccination programs, and the administration of in-feed sub-therapeutic antibiotics and antimicrobials. Persistent use of subtherapeutic antimicrobials have been implicated in the emergence of resistant-bacterial strains that colonize the gastrointestinal tract of poultry and other food animals. This have led to increasing pressure from consumers on the animal and poultry industries, to halt the use of in-feed antimicrobials. Consequently, federal agencies have created regulations that limit their use in poultry and livestock feed, thereby fostering significant research efforts to identify non-antibiotic alternatives.
To date a variety of nutraceuticals and biogenics such as probiotics, prebiotics, synbiotics, organic acids, essential oils, and antimicrobial peptides [7-16] have been investigated as nonantibiotic growth- and health-promoting feed additives in animal production. Nutraceuticals are medicinal food (or feed) ingredients that are included in diets to improve human or animal health [17]. Biogenics are health-promoting biomolecules that are produced from life processes [18]. However, research results indicate variability in the effectiveness of these products. Spray-dried animal plasma (SDAP) is a relatively new biogenic that have been successfully used in recent years to combat enteric infections in weanling piglets in the swine industry [19]. Documented beneficial effects of dietary SDAP during the weaning phase of the piglet’s life include enhanced intestinal development, improve health, and growth performance [20]. In comparison, studies documenting the feasibility of using SDP as an immunoenhancing feed additive in poultry is minimal.
The aim of this review is to highlight the research findings on the use of SDP in promoting growth and immune function in various animal species, and consequently explore its potential use in poultry production.
Manufacture of SDAP and mode of action
SDAP is a protein-rich product obtained from the industrial fractionation of blood from healthy animals [21,22] (Figure 1). Blood is collected in the abattoir during the slaughter of swine or cattle into containers containing an anticoagulant, and then shipped by refrigerated transport to the processing facility. Here, the blood is subjected to cellular fraction by centrifugation, after which the separated plasma is collected and concentrated by vacuum evaporation, filtration with inverse osmotic membranes, or by ultrafiltration. The concentrated plasma is then spray-dried, thus yielding the product known as “spray-dried animal plasma”.
Figure 1:
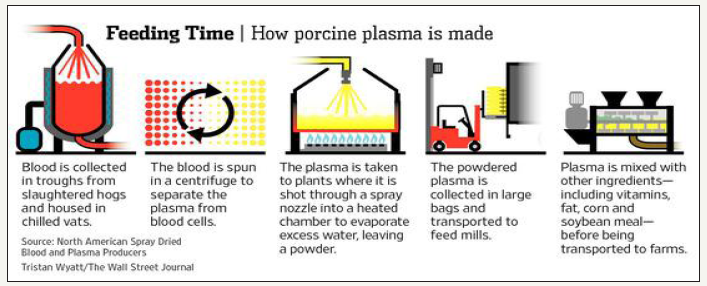
During spray drying, plasma proteins are exposed to high temperatures for a very short period (HTST). This HTST process have an advantage over conventional drying in that the proteins are not denatured with this procedure, thereby preserving most of their biological activity [19,23]. The mode of action of SDAP appears to be by reducing the attachment, adhesion, and replication of pathogens, facilitating tissue repair, or reducing the overall inflammatory response both systemically or locally [19,24]. Although information is scanty regarding the efficacy of SDAP as a health-promoting feed additive in Poultry, considerable evidence has been gathered from research studies conducted with other food, laboratory, and pet animals, particularly swine.
Effect(s) of dietary SDAP in swine production
SDAP was first presented as a protein source for use in piglet’ diets in the late 1980s. Since then, there have been many studies done to assess its benefits particularly during the weaning phase of a piglets’ life [25]. The weaning phase is frequently investigated in this regard because it has been established to be a stressful time due to the piglet switching from liquid to solid diet, in addition to a decrease in nursing phase. This stressful phase negatively impacts the pig’s ability to resist infections, thereby allowing the development of various health problems. A primary indicator of the presence of an infection during the weaning phase is an increase in the level of inflammatory cytokines, which can subsequently compromise intestinal barrier function and permeability [26]. For instance, Gao et al. [27] observed that dietary inclusion of unprocessed or autoclaved SDAP at 10% level decreased proinflammatory and anti inflammatory cytokine levels, upregulated antioxidant system in serum and intestine, and promoted intestinal development in 21-d old piglets, thereby resulting in improved growth performance. In another study, dietary SDAP was found to reduce TNF-α in the colon, and also reduced IFN-γ in the ileum and colon of weaning piglets over a seven-day period [25]. TNF-α is a necrosis factor that can induce tumor cell apoptosis and cachexia, while IFN-γ is a cytokine that in inducing and modulating an array of immune responses, primarily inflammation [28,29]. These finding demonstrate the benefits of SDP in swine during stressful production phases.
Effect(s) of Dietary SDAP in ruminant production
Dairy cattle go through intermittent stressful periods during their life cycle. Quigley & Wolfe [30] investigated the effects of SDAP in calf milk replacer on health and growth of dairy calves. Male Holstein calves (120) were randomly assigned to three dietary treatments. Treatment 1 consisted of calves fed milk replacers formulated to contain whey protein concentrate as the primary protein source. Treatments 2 and 3 consisted of calves given milk replacers formulated to contain 5% bovine or porcine spray dried plasma, respectively. Results indicated that calves given SDAPsupplemented diets had a lower rate of diarrhea, improved feed efficiency, and reduced morbidity and mortality. In another study, Lee et al. [31] compared the effects of dietary SDAP and blood meal (BM) on production parameters and blood profile of dairy cows during the transition from gestation to early-lactation period. They found that dietary SDAP supplementation reduced muscle catabolism in experimental cows, probably by serving as a source of highly digestible protein, thereby increasing the supply of amino acids to the tissues [31]. It was concluded that SDAP at 400g/d increased milk and milk component yields without an increase in feed intake.
Effect(s) of dietary SDAP on fish health
Aquaculture is a rising business as the global population continues to grow. The increasing demand for fish has fostered the development of intensive indoor fish rearing systems in which large fish populations are reared in indoor tanks. To mitigate the incidence of disease outbreak, it has become necessary for researchers to find non-antibiotic strategies that can be applied to maintain healthy fish populations. Araújo et al. [32] evaluated the effect of SDAP on the growth performance of Nile tilapia, and its potential to improve fish health under cold-induced stress. Results indicated that dietary SDAP supplementation improved growth performance, intestinal health, hematological profile, and stress resistance in Nile tilapia. Gibert et al. [33] also investigated the potential of SDAP to improve immunity in gilthead sea bream fingerlings. In this study, the fingerlings were split into 3 groups and given isonitrogenous (CP=51.2%) and isolipidic (fat=12.4%) grower diets in which high quality fish meal was replaced with 0, 3, or 6% porcine spray-dried plasma over a period of 60 d. Results indicated that inclusion of porcine spray-dried plasma in gilthead sea bream feed enhanced intestinal and serum innate immune function, activity of antioxidative stress enzymes of the intestine, and promoted growth performance.
Effect(s) of dietary SDAP on laboratory animals
A study was done by Pérez Bosque et al. [34] to investigate whether dietary supplementation of SDAP and Ig concentrate (IC) could modulate cytokine expression and inflammatory mediators in Wistar-Lewis rats challenged with Staphylococcus aureus enterotoxin B (SEB). The rats were randomly assigned to 3 dietary treatments. The control treatment consisted of rats given standard milk protein diet. Treatments 2 and 3 comprised of rats given the control diet supplemented with SDAP (at 8% level) or IC (at 1.5% level). Results showed that both SDAP and IC were effective in preventing the SEB-induced increase in pro-inflammatory cytokines in the Peyers patch, mucosa, and serum. The protective effects of plasma supplements on intestinal inflammation in this study, involved modulation of intestinal cytokines, characterized by an increased expression of anti-inflammatory cytokines [34]. In a subsequent experiment, Pérez-Bosque and co-workers observed that the anti-inflammatory effects of SDAP involve the regulation of transcription factors and adhesion molecules that reduce intestinal cell infiltration and the degree of the inflammatory response.
Effect(s) of dietary SDAP on pet animals
A few studies have documented the beneficial effects of dietary SDAP in pets, specifically dogs and cats. Quigley et al. [35] conducted three trials in which 22 beagles were fed diets into which different percentages of SDAP have been added. In Trial 1, dry extruded dog food kibbles were coated with 5% tallow, 2% commercial flavor, and 0 or 2% SDAP (as-fed basis). In Trial 2, commercially available dry dog food, previously coated with fat and flavor were coated with 0 or 2% SDAP. In Trial 3, SDAP (0, 1, 2, or 3%) was blended with other ingredients and extruded (as-fed basis). Results indicated that SDAP improved digestion and decreased fecal output. In another study [36], the gastrointestinal tract of adult dogs (9 beagles; 3 replicates with each consisting of 3 beagles) and 12 mixed breed cats (4 replicates with each consisting of 3 beagles) fed diets containing spray-dried porcine plasma (SDPP) or porcine immunoglobulins concentrate (PIC) were evaluated for immunological functionality. The control diet was a commercial diet coated with fat and digest, and the two experimental diets were similar to the control diet, but they included 10 of either SDPP or PIC/kg of diet. Results obtained suggested that plasma immunoglobulins in SDPP or PIC partially resisted digestion in the gastrointestinal tract of experimental dogs and cats and maintained part of their immunological functionality. Survival of the plasma immunoglobulins was supported by the identification of bioactive fragment Fab (containing the antigen combining site) in feces. Consequently, it was concluded that plasma immunoglobulins retain functionality after exiting the gut of dogs and cats.
Implications for poultry health and production
Significant research evidence indicates that dietary SDAP are beneficial to food, laboratory, and pet animals by promoting growth and resistance to diseases. This beneficial effect of SDAP is pronounced during stressful periods (or phases) of the animal life cycle, such as the weaning phase for pigs and calves, during lactation period when there is very high demand on the animal to produce milk for sale and nurse their babies, during disease situations, and under conditions of intensive rearing. Consequently, poultry scientists have begun studies to evaluate the possibility to harness the beneficial effects of SDAP on growth performance and disease resistance in birds.
Studies conducted by the Kansas State University to evaluate the effects of SDAP on broiler growth revealed that SDAP improved growth performance [37]. Jamroz et al. [38] also reported that dietary supplementation of SDAP to birds’ diet gave comparable efficacy to soybean meal in improving the body weight of chicks, and enhancing the retention of essential minerals such as Na and K. Furthermore, Bregendahl et al. [39] conducted a study to evaluate the effect of environmental sanitary condition on the efficacy of spray-dried plasma from porcine to promote growth performance in broiler chickens. Carcass characteristics were also evaluated. In Experiment 1, 480 chickens were raised for 42 days on fresh litter, while Experiment 2 birds were raised on the soiled litter from Experiment 1. Results showed that porcine spray-dried plasma did not affect growth performance or carcass characteristics in Experiment 1, but improved growth rate, feed conversion, breast meat yield, and flock uniformity of broilers in Experiment 2 that had the more unsanitary conditions. Overall, broilers that consumed diets containing porcine spray dried plasma had lower mortality, faster rate of body weight gain, and higher uniformity (P<0.05) compared to those fed control diets. The implication of these findings is that SDAP have the potential to promote growth, and perhaps enhance disease resistance, particularly when birds are raised in a relatively unsanitary condition.
The United States has one of the world’s largest poultry industry, which encompasses meat and egg production. In the intensive rearing systems typically used to rear commercial poultry, birds experience biological/production stressors such as heat, cold, stocking density, restraint, cooping, and shackling, and nutritional stressors that include fasting, feed restriction, and dietary protein deficiency [40,41]. It is therefore imperative for poultry scientists to further explore the use of SDAP in poultry diets, particularly to define its optimum dietary inclusion level for enhancing gut health and disease resistance in various poultry species (turkeys, ducks, quails, geese, pheasants) raised in different environmental conditions, without compromising growth performance.
Significant research evidence presented in this review indicates that dietary SDAP is beneficial to food, laboratory, and pet animals by promoting growth and resistance to diseases. This beneficial effect is more pronounced during stressful periods (or phases) of the animal life cycle, such as during the weaning phase for pigs and calves, during lactation period when there is very high demand on the cow to produce milk for sale and nurse their babies, and under conditions of intensive rearing and disease outbreaks. These findings in addition to the minimal research results obtained from studies with chickens, indicate that dietary inclusion of SDAP will likely promote growth, gut health, and disease resistance in poultry species, particularly when reared under unsanitary or intensive rearing conditions that impart stressors.
Acknowledgement
This review work was funded by NIFA through the Agricultural Research Program at North Carolina Agricultural and Technical State University (Evans-Allen Program, project number NC.X-305- 5-17-120-1).
References
- Cheng WC, Kuo CW, Chi TY, Lin LC, Lee CH, et al. (2013) Investigation on the trend of food-borne disease outbreaks in Taiwan (1991-2010). J Food and Drug Analysis 21(3): 261-267.
- Grass JE, Gould LH, Mahon BE (2013) Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998-2010. Foodborne Pathog Dis 10(2): 131-136.
- Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, et al. (2013) Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998-2008. Emerging infectious diseases 19(3): 407-415.
- Ventola CL (2015) The antibiotic resistance crisis: Part 1: causes and threats. Pharmacy and Therapeutics 40(4): 277-283.
- Sneeringer S, MacDonald JM, Key N, McBride WD, Mathews K (2015) Economics of antibiotic use in U.S. livestock production. United States Department of Agriculture, Economic Research Report, USA.
- United States Department of Agriculture (2015) National Agricultural Statistics Service: USDA poultry production data.
- Fijan S (2014) Microorganisms with claimed probiotic properties: An overview of recent literature. Int J Environ Res Public Health 11(5): 4745-4767.
- Cheng G, Hao H, Xie S, Wang X, Dai M, et al. (2014) Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front Microbiol 5: 217.
- Smialek M, Burchardt S, Koncicki A (2018) The influence of probiotic supplementation in broiler chickens on population and carcass contamination with Campylobacter spp.-Field study. Res Vet Sci 118: 312-316.
- Gadde U, Kim WH, Oh ST, Lillehoj HS (2017) Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev 18(1): 26-45.
- Saminathan M, Chin SC, Kalavathy R, Norhani A, Wan HY (2014) Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. Journal of the Science of Food and Agriculture 94(2): 341-348.
- O’Bryan CA, Pendleton SJ, Crandall PG, Ricke SC (2015) Potential of plant essential oils and their components in animal agriculture - in vitro studies on antibacterial mode of action. Front Vet Sci 2: 35.
- Amerah AM, Mathis G, Hofacre CL (2012) Effect of xylanase and a blend of essential oils on performance and Salmonella colonization of broiler chickens challenged with Salmonella Heidelberg. Poultry Science 91(4): 943-947.
- Shuai W, Xiangfang Z, Qing Y, Shiyan Q (2016) Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci 17(5): 1-12.
- Bechinger B, Gorr SU (2017) Antimicrobial peptides: Mechanisms of action and resistance. J Dent Res 96(3): 254-260.
- Choi SC, Ingale SL, Kim JS, Park YK, Kwon IK, et al. (2013) Effects of dietary supplementation with an antimicrobial peptide-P5 on growth performance, nutrient retention, excreta and intestinal microflora and intestinal morphology of broilers. Anim Feed Sci Technol185(1-2): 78- 84.
- Kalra EK (2003) Nutraceutical-definition and introduction. AAPS Pharm Sci 5(3): 27-28.
- Mallela K (2010) Pharmaceutical biotechnology-concepts and applications. Human Genomics 4(3): 218-219.
- Perez Bosque A, Polo J, Torrallardona D (2016) Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porcine Health Management 2: 16.
- Müller LKF, Paiano D, Gugel J, Lorenzetti WR, Santurio JM, et al. (2018) Post-weaning piglets fed with different levels of fungal mycotoxins and spray-dried porcine plasma have improved weight gain, feed intake and reduced diarrhea incidence. Microbial Pathogenesis 117: 259-264.
- Heinz G, Hautzinger P (2007) Meat processing technology for smallto- medium scale producers. Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific, RAP Publication, pp. 1-470.
- Gara T, Newman J, Kelsey G (2014) Questions arise in the spray dried blood business WSJ blog, Dow Jones Institutional News.
- Borg BS, Campbell JM, Russel LE, Rodríguez C, Ródenas J (2002) Evaluation of the chemical and biological characteristics of spray-dried plasma protein collected from various locations around the world. Proc Am Assoc Swine Vet 33: 97-100.
- Campbell JM, Crenshaw JD, Russell LE, Hayes SK (2008) Influence of dietary plasma proteins on supporting animal immunity systems.
- Peace RM, Campbell J, Polo J, Crenshaw J, Russell L, et al. (2011) Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J Nutr 141(7): 1312-1317.
- Campbell J, Polo J, Russell L, Crenshaw J (2010) Review of spraydried plasma’s impact on intestinal barrier function. Livestock Science133(1-3): 239-241.
- Gao YY, Jiang ZY, Lin YC, Zheng CT, Zhou GL, et al. (2011) Effects of spraydried animal plasma on serous and intestinal redox status and cytokines of neonatal piglets. J Anim Sci 89(1): 150-157.
- Tau G, Rothman P (1999) Biologic functions of the IFN-γ receptors. Allergy 54(12): 1233-1251.
- Pfeffer K (2003) Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine & Growth Factor Reviews 14(3-4): 185- 191.
- Quigley JD, Wolfe TM (2003) Effects of spray-dried animal plasma in calf milk replacer on health and growth of dairy calves. J Dairy Sci 86(2): 586-592.
- Lee C, Tebbe AW, Campbell JM, Weiss WP (2018) Effects of spray-dried plasma protein product on early-lactation dairy cows. J Dairy Sci 101(7): 6019-6031.
- Raújo EP, Carvalho PLPF, Freitas JMA, Silva RL, Rocha MKHR, et al. (2017) Dietary spray-dried plasma enhances the growth performance, villus: crypt ratio and cold-induced stress resistance in Nile tilapia (Oreochromis niloticus). Aquaculture 479: 675-681.
- Gisbert E, Skalli A, Campbell J, Solovyev M, Rodríguez C, et al. (2015) Spray-dried plasma promotes growth, modulates the activity of antioxidant defenses, and enhances the immune status of gilthead sea bream (Sparus aurata) fingerlings. J Anim Sci 93(1): 278-286.
- Pérez Bosque A, Miró LS, Polo J, Russell L, Campbell J, et al. (2010) Dietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in rats. J Nutr 140(1): 25-30.
- Quigley IJD, Campbell JM, Polo J, Russell LE (2004) Effects of spray-dried animal plasma on intake and apparent digestibility in dogs. J Anim Sci 82(6): 1685-1692.
- Rodriguez C, Blanch F, Romano V, Saborido N, Rodenas J, et al. (2007) Porcine immunoglobulins survival in the intestinal tract of adult dogs and cats fed dry food kibbles containing spray-dried porcine plasma (SDPP) or porcine immunoglobulin concentrate (PIC). Anim Feed Sci Technol 139(3-4): 201-211.
- Campbell JM, Russell LE, Crenshaw JD, Behnke KC, Clark PM (2006) Growth response of broilers to spray-dried plasma in pelleted or expanded feed processed at high temperature. J Anim Sci 84(9): 2501- 2508.
- Jamroz D, Wiliczkiewicz A, Orda J, Kuryszko J, Stefaniak T (2012) Use of spray-dried porcine blood by-products in diets for young chickens. J Anim Physiol Anim Nutr 96(2): 319-333.
- Bregendahl K, Ahn DU, Trampel DW, Campbell JM (2005) Effects of dietary spray-dried bovine plasma protein on broiler growth performance and breast-meat yield. J Appl Poult Res 14(3): 560-568.
- Scanes CG (2016) Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult Sci 95(9): 2208-2215.
- Marco Ramell A, de Almeida AM, Cristobal S, Rodrigues P, Roncada P, et al. (2016) Proteomics and the search for welfare and stress biomarkers in animal production in the one-health context. Mol Bio Syst 12(7): 2024-2035.
© 2018 Yewande O Fasina. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






