- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Peter Xiao, John He, Colin Pouton and Zhi Cheng Xiao*
SPeter Xiao, John He, Colin Pouton and Zhi Cheng Xiao*
iRiccorgpharm Pty Ltd, Australia
*Corresponding author: Zhicheng Xiao, iRiccorgpharm Pty Ltd, Melbourne, 3800, VIC, 68 Price Rd, Mount Waverly, Vic 3165, Australia
Submission: October 05, 2018;Published: October 15, 2018

ISSN: 2576-9162 Volume5 Issue2
Abstract
Background: Various countries in the world have been using phytochemical agents derived from traditional plants in order to manage and control disease outbreaks in production animal. Poultry industry are also looking into alternatives to current industry feed ingredients and phytogenic compounds are one such alternatives. In the “western” world, even though these agents haven’t been used in animal husbandry, however plenty have been used for human pharmaceutical products. The lack of residue and toxicity data has been preventing phytogenic compounds to be used to combat disease in poultry. Current studies done by IRP focuses on the general health of broiler chickens by administering feed, with the natural compound that have been identified, such as, Berberine, Ursolci Acid and Piceid in different levels.
Methods and Results: 64 pens of 20 male broiler chickens with be used in this experiment. The treatments will be divided into eight (8) blocks and each block will be randomized. Southern Poultry Feed and Research, Inc. will conduct the randomization procedure for pen assignment for treatments and blocks. Source data will include the pen diagram and treatment assignment.
Conclusion: The actual results of the trial are submitted to the sponsors, Where the accurate data were obtained through trials that were conducted according to the procedures described in the protocol. No adverse reaction was observed in the test treatments and the data was collected in way that will not misrepresent the true effects, and the sponsor have obtained all relevant data.
Abbreviations: BW: Bodyweight; FCR: Feed Conversion Ratio; LC/MS-MS: Liquid Chromatography Tandem-Mass Spectrometry; LLOD: Lower Limit of Detection; LLOQ: Lower Limit of Quantification
Introduction
Antibiotics have been used in feed for animals with the intention of preventing diseases and increasing the feed conversion efficiency, rather than treatment of sick animals [1]. A recent investigation on antimicrobial resistance states that over 70% of medically important antibiotics in the US are sold for use in animals. This has contribute to antimicrobial resistance and the development of ‘superbugs’, which has compelled action to be taken in order to curb this predicament, one such method is the necessity to replace antibiotics in animal production [2]. Phytogenic compounds provide one such options, as they have become increasingly studied in recent years, and can act as a substitutes to antibiotics in feed for the livestock industry [3]. Therefore, in an attempt to progress the economic state of livestock industry and to combat against antimicrobial resistance, the identification and improvement of alternatives that do not hinder productivity is crucial [4].
In 1920 a pentacyclic triterpenoid compound has been identified to reside in peels of fruits, such as the epicuticular waxes of apples, as well as in herbs and spices like rosemary and thyme [5,6]. The compound is known as Ursolic acid (sometimes referred to as urson, prunol, malol, or 3-beta-3-hydroxy-urs-12- ene-28-oic-acid), and is present in various plants, such as Mirabilis jalapa, as well as in numerous fruits and herbs that are used daily (e.g. prunes, basil, peppermint, hawthorn, elder flower, bilberries, oregano, apples, rosemary, thyme, cranberries, and lavender) [7,8]. Ursolic acid and related compounds are found in large quantities in apple peels [9].
Berberine is an isoquinoline quaternary alkaloid found present in many plants such as Hydrastis canadensis (goldenseal) and B. vulgaris (barberry) and Coptis chinensis (Chinese goldthread) and has been recognized as the major active component [10,11]. Humans have been using Berberine for thousands of years in traditional medicines for the treatment of intestinal maladies [11,12]. Positive outcomes for poultry trials using Berberine to control Fowl Cholera, Coccidiosis and Necrotic Enteritis have been the researchers results of many researchers in the past few years [13].
Piceid which is found in the bark of Picea sitchensis or isolated from Fallopi Japonica, the Japanese knotweed (syn. Polygonum cuspidatum) [14]. It is a stilbenoid glucoside and is a major resveratrol derivative in grape juices [15]. Piceid fermented by Aspergillus oryzae can produce resveratrol [16]. This latter species produces a piceid-b-D-glucosidase. cis-resveratrol forms the glucoside cis-piceid, while trans-resveratrol can form the glucoside trans-piceid. Trans-resveratrol-3-O-glucuronide is one of the two metabolites of trans-piceid in rat. The prevention of aberrant crypt foci in mice uses resveratrol glucoside which is from transgenic alfalfa.
The effects on performance and intestinal health, in poultry, of the above mentioned compounds, Ursolic acid, Berberine and Piceid, is the aim of this study. The targeted animal safety is also a subject to be evaluated in this trial.
Source of material and animals
Compounds: Phytogenic compounds including Berberine Chloride, Ursolic Acid and Piceid were sourced from the JiaHe (Shaanxi, China).
Birds: One-day-old male Cobb X Cobb chicks will be obtained from Cobb-Vantress hatchery, Cleveland, GA. 1200 chicks will be allocated to the study. All chicks will be spray vaccinated with a commercial coccidia vaccine at the recommended level prior to placement. Twenty male broiler chicks will be placed in each pen. Accountabilities of all test animals and any extra birds will be recorded on animal disposition form. The birds will be sexed at the hatchery. The breeder flock history and vaccination record at the hatchery will be recorded. The broilers will not be vaccinated at the farm. Bird weights by pen will be recorded on Days 0, 21, 35, and 42.
The experiment will consist of 64 pens of 20 male broiler chickens. The treatments will be replicated in eight (8) blocks; the eight (8) treatments will be randomized within each block. A randomization procedure for pen assignment for treatments and blocks will be done by Southern Poultry Feed and Research, Inc. The pen diagram and treatment assignment will be included with the source data. The test house is divided into pens of equal size, arranged along a central aisle. Each pen is 20.25 (4.5’ X 4.5’) sq. ft. and has 2 feet high side walls with bottom 1/2 foot being of solid wood to prevent bird migration. The pens will be prepared for use in the study according to SPR SOP. All flooring of each pen will have approximately 4 inches used litter shavings. The pen will be the experimental unit. All pens will be numbered consecutively and identified on pen cards. The temperature of the building will be monitored. Environmental conditions during the trial (temperature) will be appropriate (optimum) to the age of the animals. Illumination will be provided by fluorescent bulbs placed above the pens. The lighting scheme will be 24 hours of light per day from day 0 to day 42.
Standard floor pen management practices will be used throughout the experiment. Animals and housing facilities will be inspected twice daily, observing and recording the general health status, constant feed and water supply as well as temperature, removing all dead birds, and recognizing unexpected events. Birds found dead during the study will be noted on the Daily Mortality Record, and will not be replaced. Pen number, the date of mortality, sex, weight, and diagnosis will be recorded. All birds and feed will be buried in SPR’s pit as described in SPR SOPs. Records of disposition will be included in the source data.
Diets: The diets will be provided ad libitum in one tube-type feeders per pen. From day 0 until day 7, feed will also be supplied on trays, directly placed on the litter. Water will be provided ad libitum from one Ziggity nipple line per pen (six available nipples/ pen).
Southern Poultry Research, Inc. will provide all feeds (Table 1). All feeds will be manufactured at SPR Feed Mill. Quantities of all basal feed and test products used to prepare treatment batches will be documented. Each batch of feed will be mixed and bagged separately. Each bag will be identified with the study number, date of mix, type of feed, and the correct treatment number. Complete records of feed mixing, and test article inventories will be maintained.
Table 1:Diet.
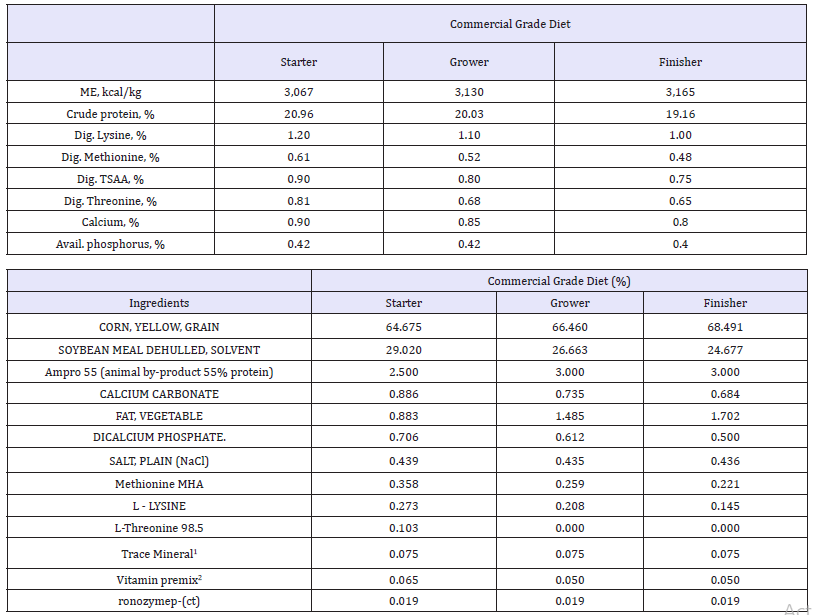
The main ingredients used were corn, soybean meal and animal by product.
1Vitamin mix provided the following (per kg of diet): thiamin•mononitrate, 2.4mg; nicotinic acid, 44mg; riboflavin, 4.4mg; D-Ca pantothenate, 12mg; vitamin B12 (cobalamin),12.0μg; pyridoxine•HCL, 4.7mg; D-biotin, 0.11mg; folic acid, 5.5mg; menadione sodium bisulfite complex, 3.34mg; choline chloride, 220mg; cholecalciferol, 27.5ug; trans-retinyl acetate, 1,892ug; all-rac α tocopheryl acetate, 11mg; ethoxyquin, 125mg.
2Trace mineral mix provided the following (per kg of diet): manganese (MnSO4•H2O), 60mg; iron (FeSO4•7H2O), 30mg; zinc (ZnO), 50mg; copper (CuSO4•5H2O), 5mg; iodine (ethylene diamine dihydroiodide), 0.15mg; selenium (NaSe03), 0.3mg.
The basal feed did not contain any probiotic/ prebiotic feed additives, NSPases, coccidiostats or antibiotic growth promoter. All diets contained phytase.
Starter feed will be fed from DOT 0 to 21. On DOT 218, nonconsumed starter will be weighed by pen and discarded. Grower feed will be issued and fed until DOT 35. On DOT 35, non-consumed grower will be weighed by pen and discarded. WD feed will be issued and fed until DOT 42. On DOT 42, non-consumed WD will be weighed by pen and discarded. Treatment feed samples (~150g each) were collected and blended: one each from the beginning, middle, and end of each batch of treatment diet. Samples will be retained at SPR until directed to ship or discarded 2 months post submission of report.
<Histological samples
On the day of study completion (D42), five birds from each pen were humanly euthanized and upper, mid and lower gut sections plus liver lobe were collected and stored in neutral buffered formalin. Theses samples were shipped to Veterinary Diagnostic Pathology, LLC for analysis.
Data entry and analysis
Source data will be entered with indelible ink. Entries will be legible, signed or initialed, and dated by the person making the observation entry. Each sheet of source data will be signed by the person(s) attributed to the data. Any mistakes or changes to the source data will be initialed and dated and a correction code or statement added as to why the changes were made. Means from (Day 0-21, 0-35, and 0-42), for pen weight gain, feed consumption, and feed conversion for each feed period will be calculated. The original source data sheets and the final report will be sent to Sponsor. An exact copy of the file and the final report will be retained at Southern Poultry Feed and Research, Inc.
Assessment of effects
Twice daily observations were recorded during the study for general flock condition. Observations included were the availability of feed and water, temperature control, and any unusual conditions. The birds were watched closely for any abnormal reactions. Feed intake, bodyweight (BW) and feed conversion ratio (FCR) were recorded and compared between groups to determine treatment effects. Bodyweight was recorded on day 0 and 42. The mean initial weight of the chicks for all groups was recorded as not significantly different. FCR was calculated by the following formulae [17]:
Intestinal pathology and histology
Duodenum, some with pancreas, jejunum, and ileum from chickens at 42 days of age, were submitted fixed in formalin for histologic examination. 2mm sections of tissue were trimmed from the submitted tissue, placed in cassettes, and processed for paraffin-embedded 5 μm sections stained with hematoxylin and eosin (H&E). All intestinal sections were kept intact in circular form to ensure uniformity of assessment. Tissues were examined microscopically for lesions and for parasites. A lesion panel was developed for each tissue, and lesions were semi-quantitatively scored for severity per 0, normal; 1, minimal severity; 2, mild severity; 3, moderate; 4, marked and 5, severe. Coccidia if present were identified to species (if possible) and scored according to previous work [18,19].
For each bird, a coccidia index was calculated by summing the coccidia scores from each section of intestine. A cumulative pathology index was calculated by summing all lesion scores for all sections of intestine. The total enteritis index was calculated by subtracting the coccidia index from the cumulative lesion index, leaving a number representing inflammation and repair. On the day of study completion (day 42), five (5) birds from each pen were humanely euthanized and upper, mid and lower gut sections plus liver lobe were collected and stored in neutral buffered formalin. These samples were shipped to Veterinary Diagnostic Pathology, LLC for microscopic lesion analysis. Lesions were scored for severity as 0, lesion absent or within normal; 1, minimal severity; 2, mild severity; 3, moderate severity; 4, marked severity; 5, severe. Lesion scores were recorded in a spreadsheet. A hepatitis index was calculated by summing all lesion scores from each liver.
Results
Feed intake, FCR and average weight gain
Table 2:General effects of phytogenic compounds on poultry.
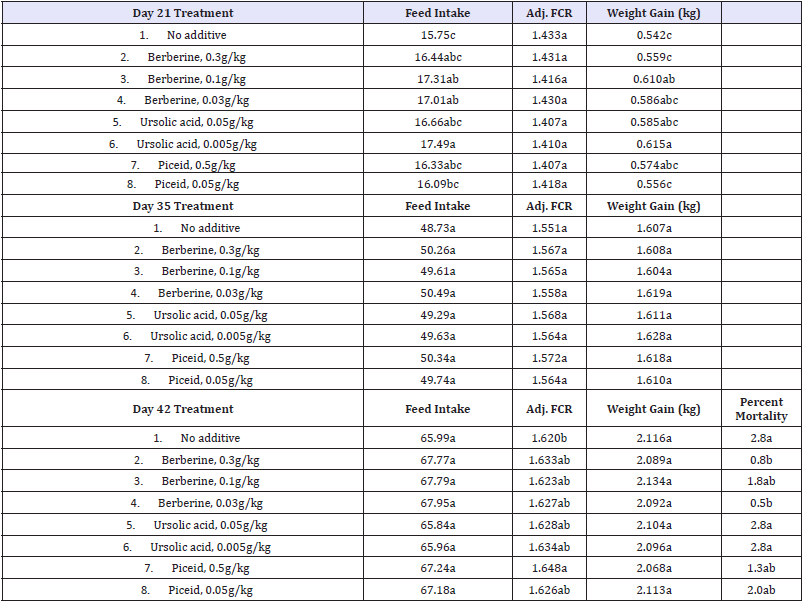
Table 2 summarizes the general effects of three phytogenic compounds in poultry. All birds appeared normal and no adverse effects or unanticipated events occurred. This is reflected in the results showing no ill effects of the compounds on feed intake, FCR or average weight gain. In fact, a slight improvement in FCR when a phytogenic was added to the feed was found when compared to the control group.
Intestinal pathology and liver histology
The effect of the three phytogenic compounds on intestinal pathology is summarized (Berberine, Table 3; Ursolic acid, Table 4; Piceid, Table 5). Livers in control and treatment chickens had mild lesions without differences observed in the liver lesion index among groups. These changes included mild lymphocytic hepatitis in the portal regions, and extramedullary hematopoiesis, within normal limits for a production environment.
Table 3:Liver pathology of Berberine treated birds.
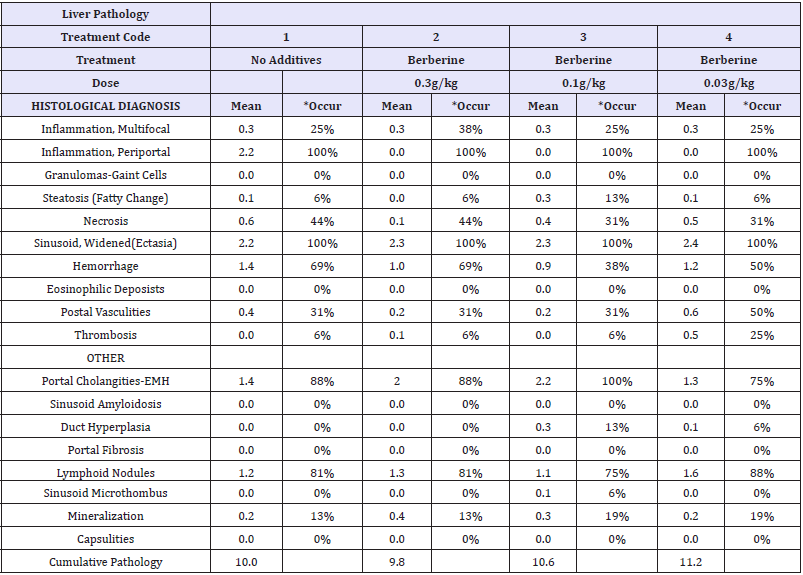
Table 4:Liver pathology of Berberine treated birds.

Table 5:Liver pathology of Piceid treated birds..

Discussion
Phytogenic compounds have the potential to contribute to general health management and disease control of poultry [1]. They hold advantages in that they can be administered from day-of-hatch and are relatively easy to translate to commercial use. Thousands of phytogenic compounds have also been identified as inhibitory towards microorganisms [20]. However, the limited in vivo data and sometimes contradictory findings, suggest it cannot be univocally determined what contribution phytogenic feed ingredients may offer. This study hopes to provide further insight into the feasibility of phytogenic compounds as feed ingredients for the poultry industry by conducting a general target poultry safety study with three phytogenic compounds, and for the first time, assessing the tissue residue of Berberine in poultry to aid regulatory institutes such as the APVMA and FDA in setting the optimal method for Berberine use in poultry.
Our study demonstrates that Berberine, Ursolic Acid and Piceid are not harmful even at the highest concentration tested. In fact, the results show an improvement in FCR although conclusions cannot be drawn due to the lack of statistical significance. Nevertheless, there has been accumulating evidence that phytogenics can modulate the gut microbiota to confer beneficial effects. This is reflected in the cumulative pathology scores, where the phytogenic compounds markedly reduced enteritis and coccidia lesions, and further supported by recent studies reporting the anticoccidial effect of Berberine and its activity against Clostridium Perfringens in poultry, antimicrobial activity of Ursolic Acid in irradiated fresh poultry, improved poultry performance of birds treated with Piceid. However, despite the apparent positive effects of the phytogenic compounds, the gastrointestinal histologic lesions identified were within normal limits for broiler chick in a production environment, as were the liver histological lesions. Therefore, while no conclusions can be made regarding the activity of the compounds tested, there is evidence to presume that they pose no harm to commercial broilers.
Conclusion
This study shows that Berberine, Ursolic Acid and Piceid, caused no discernible adverse effect in poultry when administered as an ingredient in-feed. Interestingly, the study results suggest there is potential for better production performance and general health of poultry with the tested phytogenic compounds showing slight improvement in performance and intestinal pathology. However, further studies are still required to understand the function mechanism of these compounds.
Acknowledgement
This research was conducted with the help of the Southern Poultry Research, Inc. Georgia, USA and Veterinary Health Research Ltd, Armidale, NSW, Australia. We are thankful to Greg Mathis, Charles Hofacre and Bruce Chick for their assistance and expertise. Any errors are our own and should not tarnish the reputations of these esteemed professionals.
Funding
This research was supported by iRiccorgpharm Pty Ltd., in its initiative to reduce antibiotic usage in livestock.
Competing and Conflicting Interests
This report may be used as supporting data for iRiccorgpharm Pty Ltd phytogenic dossier for regulatory approval..
References
- Yang C, Chowdhury MA, Huo Y, Gong J (2015) Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens 4(1): 137-156.
- Sanderson CE, Fox JT, Dougherty ER, Cameron ADS, Alexander KA (2018) The changing face of water: A dynamic reflection of antibiotic resistance across landscapes. Front Microbiol 9: 1894.
- Rousham EK, Unicomb L, Islam MA (2018) Human, animal and environmental contributors to antibiotic resistance in low-resource settings: integrating behavioural, epidemiological and One Health approaches. Proc Biol Sci 285(1876).
- Hrvatin V (2017) Combating antibiotic resistance: New drugs or alternative therapies? CMAJ 189(37): E1199.
- Abe F, Yamauchi T, Nagao T, Kinjo J, Okabe H, et al. (2002) Ursolic acid as a trypanocidal constituent in rosemary. Biol Pharm Bull 25(11): 1485- 1487.
- Rowe EJ, Orr JE (1949) Isolation of oleanolic acid and ursolic acid from Thymus vulgaris, L. J Am Pharm Assoc Am Pharm Assoc 38(3 Pt. 1): 122- 124.
- Lv Y, Tahir II, Olsson ME (2016) Factors affecting the content of the ursolic and oleanolic acid in apple peel: influence of cultivars, sun exposure, storage conditions, bruising and Penicillium expansum infection. J Sci Food Agric 96(6): 2161-2169.
- Zerin T, Lee M, Jang WS, Nam KW, Song HY (2016) Anti-inflammatory potential of ursolic acid in Mycobacterium tuberculosis-sensitized and concanavalin A-stimulated cells. Mol Med Rep 13(3): 2736-2744.
- Jager S, Trojan H, Kopp T, Laszczyk MN, Scheffler A (2009) Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules 14(6): 2016-2031.
- Wisniewski W, Gorta T (1966) Separation of berberine, hydrastine and hydrastinine from rhizomes of Hydrastis Canadensis and from the liquid extract. Acta Pol Pharm 23(5): 455-458.
- Imanshahidi M, Hosseinzadeh H (2008) Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res 22(8): 999-1012.
- Yu C, Tan S, Zhou C, Zhu C, Kang X, et al. (2016) Berberine reduces uremia-associated intestinal mucosal barrier damage. Biol Pharm Bull 39(11): 1787-1792.
- Lv Z, Peng G, Liu W, Xu H, Su J (2015) Berberine blocks the relapse of Clostridium difficile infection in C57BL/6 mice after standard vancomycin treatment. Antimicrob Agents Chemother 59(7): 3726- 3735.
- Romero Perez AI, Ibern Gomez M, Lamuela Raventos RM, de La Torre Boronat MC (1999) Piceid, the major resveratrol derivative in grape juices. J Agric Food Chem 47(4): 1533-1536.
- Shimokawa Y, Akao Y, Hirasawa Y, Awang K, Hadi AH, et al. (2010) Gneyulins A and B, stilbene trimers, and noidesols A and B, dihydroflavonol-C-glucosides, from the bark of Gnetum gnemonoides. J Nat Prod 73(4): 763-767.
- Prokop J, Abrman P, Seligson AL, Sovak M (2006) Resveratrol and its glycon piceid are stable polyphenols. J Med Food 9(1): 11-14.
- Yan W, Sun C, Wen C, Ji C, Zhang D, et al. (2018) Relationships between feeding behaviors and performance traits in slow-growing yellow broilers. Poult Sci.
- Al Sheikhly F, Al Saieg A (1980) Role of coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis 24(2): 324-333.
- Lanckriet A, Timbermont L, De Gussem M, Marien M, Vancraeynest D, et al. (2010) The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model. Avian Pathol 39(1): 63-68.
- Kordium VA (1961) Multiplication of microorganisms from air and soil dust with special regard to phytogenic substances in greenhouses. Mikrobiol Zh 23(5): 8-12.
© 2018 Mariya Hristova. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






