- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Marekʼs Disease in A Peafowl (PavoMuticus); Pathological, Immunohistochemical and Molecular Studies
Vahid Reza Ranjbar1 and Monire Khordadmehr2*
1Clinic of Poultry Diseases, Yazd province, Iran
2Department of Pathology, University of Tabriz,Iran
*Corresponding author: MonireKhordadmehr,Department of Pathology, Faculty of Veterinary Medicine,University of Tabriz, Tabriz, Iran
Submission: June 01, 2018;Published: July 02, 2018

ISSN 2576-9162Volume4 Issue2
Abstract
Marekʼs disease, a Herpesvirus-induced lymphoproliferative disease, is known to affect chickens and other domesticated and wild birds.One of the five green peafowls (PavoMuticus) in the owner’s collection had already died. The affected bird showed non-specific clinical signs such as depression, weight loss and anorexia. At necropsy, the liver and spleen were observed diffuse enlargement accompanied by several times the normal size with white to gray in color and firm, and the cut surface was smooth. Grossly, the other visceral organs were normal. Histopathological and immunohistochemical examination were done for the affected tissues. Diffuse infiltration of the liver and spleen caused loss of normal architecture.Tumor cells are characterized by large, pleomorphic nuclei with prominent nucleoli. According to immunohistochemical findings, the majority of transformed T cells expressed CD4-/ CD8+.In addition, the immunohistochemistry for CD30 were positive. In PCR, MDV was detected by specific primers. To our knowledge, this is the first report of Marek’s disease in peafowls (PavoMuticus) which were infected naturally and had thought is refractory to this infection.
Keywords: Marekʼs disease;Herpesvirus;Green peafowl; Lymphoma; Tumor cells
Abbreviations: IHC: ImmunoHistoChemical; ICTV: International Committee on Taxonomy of Viruses;MDV: Marek’s Disease Viruses; NK: Natural killer
Introduction
Marek’s Disease (MD) is a common lymphoproliferative disease of chickens, usually characterized by mononuclear cellular infiltrates in peripheral nerves and various other organs and tissues [1]. Unlike in human medicine where the vast majority of cancers are non-infectious [2], most of the neoplastic diseases affecting poultry have a viral etiology. As per the recent classification by the International Committee on Taxonomy of Viruses (ICTV), all Marek’s Disease Viruses (MDV) serotypes are grouped together in the genus Mardivirus [3].
Virtually all chickens including game fowl are susceptible to MDV infection and tumor development [1] and are by far the most important natural host. Generally, chickens less than 16 weeks of age are most often affected. However, in late Marek’s the mortality can extend to 40 weeks of age. Affected birds are more susceptible to other diseases, both parasitic and bacterial [1]. Quail (Coturnix coturnix japonica) [4,5], turkeys [6,7], pheasants (experimental infection) (Phasianus colchicus) [8], and some species of ducks and geese (Anser albifrons) [9] are also susceptible to infection and disease. Most other avian species including sparrows, partridge, pigeons, and peafowl are probably refractory [1].
It seems that Marek’s disease virus is widespread among waterfowl without causing clinical signs. These species could be considered a reservoir for other avian species [10]. Furthermore, the incidence of MD in other avian species demonstrates the increasing host range and economic significance. Despite widespread use of vaccines and development of new methods of vaccination, MD still remains a major challenge to poultry health, particularly from the continuing increase in virulence of MDV strains. Up to now, it had thought that peafowl are probably refractory to this infection and disease. In the present paper are described clinical signs, gross morphology, histopathological (with H&E staining) and Immuno Histo Chemical (IHC) features of Marekʼs disease in an immature green peafowl (Pavo muticus) which was infected naturally.
Materials and Methods
History In a group of 5 immature green peafowls (Pavo muticus) with about 7 months old, the bird died after developing non-specific signs such as weight loss, depression, anorexia, paleness, and diarrhea. All of the birds were kept in backyard of private home after hatching which were fed with poultry food contains soybean and corn. According to the owner statement, all of the peafowls had close contact with ten adult backyard chickens (about one yearold) which showed no clinical signs or mortality at that time.
Necropsy and histological diagnosis
At necropsy, the liver and spleen were observed diffuse enlargement accompanied by several times the normal size. At first, the liver and spleen were routinely processed for possible bacterial infection. Then, gross examination of visceral organs was performed carefully and firm, and the cut surface was smooth. Necrosis was rare and occurred in the center of growing lesions. Grossly, the other visceral organs such as lung, heart, kidney, bursa, genital and alimentary systems were normal. For histopathological studies, tissue samples were fixed in 10% neutral buffered formalin, processed routinely, and embedded in paraffin wax. Sections (5μm) were stained by hematoxylin and eosin. Moreover, some sections were subjected to immunohistochemistry with primary monoclonal antibodies specific for CD4, CD8 and CD30 (Novocastra Laboratories, Newcastle, UK). Immunolabeling was detected with an avid in-biotin conjugate procedure (Novocastra Laboratories) with positive internal and external controls.
Molecular diagnosis
Molecular examination was performed for more confirmation. For this purpose, two 10μm thick sections were cut from each paraffin block and DNA was extracted by a previously described method [11]. Extracted DNA as a template with specific primers of MDV (MdCv/pp38 with forward: GTGATGGGAAGGCGATAGAA and reverse: TCCGCATATGTTCCTCCTTC) was used for PCR [12]. PCR reaction was carried out in 25μl reaction volumes, consisting of 12.5μl of Taq DNA Polymerase 2x Master Mix (Ampliqon IIII, Denmark), 20pmol of each primer, and 50-100ng of template DNA. PCR program was carried out as previously described [12]. The PCR product was analyzed on the 1.5% agarose gel.
Results
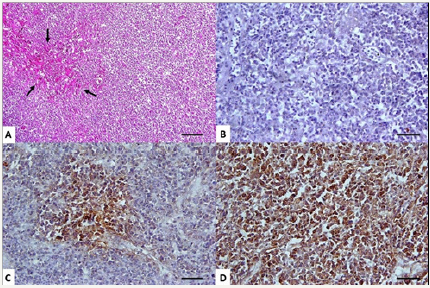
Bacteriological examination of the liver and spleen was negative. Moreover, in histological examination, there was no lesion of bacterial infection. Diffuse infiltration of the liver and spleen caused loss of normal architecture (Figure 1) and gave the surface a coarse granular appearance. Large nodular tumors also were seen in the liver. Lymphomatous lesions were similar in appearance to A-type lesions consisting of diffusely proliferating small-to-medium T lymphocytes and lymphoblasts, B cells, and macrophages, whereas plasma cells were rarely present. Tumor cells are characterized by large, pleomorphic nuclei with prominent nucleoli. According to immunohistochemical findings, the majority of transformed T cells expressed MHC-II and CD4-/ CD8+ (Figure 1) and absence of B- lymphocyte infiltration. In addition, the results of immunohistochemistry for CD30 were positive (Figure 1). DNA extraction from paraffin- embedded tissue blocks was performed successfully. MdCv/ pp38 primers were used as specific primers for detection of MDV on infected tissues. MDV was detected in paraffin-embedded liver and spleen samples of the peafowl (Figure 2).
Figure 1: Marek’s disease in a green peafowl (Pavomuticus), liver. Diffuse infiltration of the liver caused loss of normal architecture and only, a few degenerated hepatocytes were observed (A). Lymphomatous lesions were consisted of diffusely proliferating smallto- medium lymphocytes which are characterized by large, pleomorphic nuclei with prominent nucleoli; (H&E; bar=200μm). Immunohistochemical findings showed the majority of transformed T cells expressed CD4-/CD8+ (B and C, respectively) and also CD30+ (D) and absence of B- lymphocyte infiltration; (IHC; bar=30μm).
Discussion
MDV is a cell-associated Herpesvirus with lymphotropic properties similar to those of gamma Herpesviruses [1]. The disease was first described in chickens (laying hens as well as broilers), and thoroughly studied in this species. MDV is transmitted readily by direct or indirect contact between chickens by the airborne route [1]. Under commercial conditions, young chickens are most commonly exposed to MDV by contact with residual dust and dander in the growing house or by aerosolized dust from adjacent chicken houses, fomites, or personnel. Prior to the use of vaccines, MD constituted a serious economic threat to the poultry industry, causing up to 60% mortality in layer flocks and 10% condemnations in broiler flocks. Because vaccines are not 100% effective, sporadic losses still occur, but they are no longer as serious a problem. Even in susceptible chickens, infection does not always induce clinical disease, and in genetically resistant or vaccinated chickens, infection may rarely cause overt disease [1].
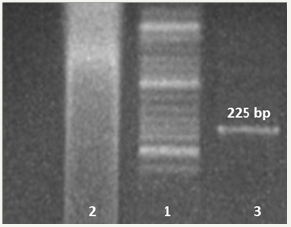
Figure 1: Agarose gel electrophoresis analysis of the PCR products with MdCv primer. 1; Orange Ruler DNA ladder. Lanes 1and 2: negative control (only added DNA in PCR without primers);
Lanes 3: PCR product of liver which shows 225bp.
In the present paper, one of five peafowls showed non-specific clinical signs and finally died (25%). While the other four peafowls and also the adult backyard chickens which had close contact with affected bird, did not show any clinical signs. In the present paper, there were no gross lesions in the peripheral nerves. While, in the affected bird, was seen lymphoma like lesions in the liver and spleen. Apparently, chickens with MD lymphomas may appear clinically normal but have extensive neo plastic involvement when euthanized, while other birds may become depressed and comatose prior to death. A few birds may apparently recover from the clinical disease, but the recovery is rarely permanent [13]. Previous studies in quail demonstrated that affected birds develop lymphomas in various visceral organs, but peripheral nerves are rarely affected. Mortality can reach 10%-20%, but deaths occurs relatively late [5]. In the affected turkey flocks, paralysis was noted in some birds, and peripheral nerves were occasionally infiltrated with lymphocytes [14]. Mortality from tumors reached 40-80% between 8-30 weeks of age.
In an older literature, challenge of the common pheasant (Phasianus colchicus) with virulent MDV induced paralysis, visceral lymphomas, and precipitating antibodies within 75-85 days post inoculation [8]. Probably, according to the clinical and necropsy findings of the present paper, as like as quail and turkeys, it seems that peafowls are not susceptible to neurological lesions of MD. Pathologic changes in MD consist mainly of nerve lesions and visceral lymphomas. Macroscopic changes are not seen in the brain, but gross enlargements can be found in spinal ganglia. Lymphomas may occur in one or more of a variety of organs and tissues. Lymphomatous lesions can be found in the gonad, lung, heart, mesentery, kidney, liver, spleen, bursa, thymus, adrenal gland, pancreas, proventriculus, intestine, iris, skeletal muscle, and skin. Visceral lymphomas are common in more virulent forms of the disease [1]. In the affected peafowl, pathologic changes were agreement with MD lesions.
In microscopic pathology of the affected peafowl, massive infiltration of lymphocytes and lymphoblasts in the liver and spleen were observed. Histopathologic changes associated with MD lymphoproliferative lesions have been described by numerous researchers who are in general agreement about the types of histologic lesions and the cells involved [1]. Generally, three types of lymphoproliferative lesions are recognized, which are referred to as type A, B and C. Type A lesions consist mostly of T cells and some B cells; this type is considered neoplastic. Lymphomatous lesions in visceral organs are (A-type lesions) consisting of diffusely proliferating small-to-medium T lymphocytes and lymphoblasts, natural killer (NK) cells, B cells, and macrophages, whereas plasma cells are rarely present (Burgess, 2004). The cellular composition of tumors is similar from one organ to another, even though the gross pattern of involvement may vary. Finally, characteristic inflammatory, type-B lesions (edema, sparse infiltrations) appeared, suggesting the occurrence of regression of A type lesions. A third lesion, C-type, consists of light infiltration of lymphocytes and plasma cells. In the present study, histopathological examination of the liver and spleen were more similar to, A type lesions which indicated massive infiltration of lymphocytes and lymphoblasts, some plasma cells and a few macrophages which these cells cause loss of normal structure of the liver and spleen. Earlier literature described that diffuse infiltration of inflammatory and lymphoproliferative cells in the liver cause loss of normal lobule architecture and often give the surface a coarse granular appearance [1]. A previous study reported a transient splenomegaly within 4-12 days post infection in some chickens which is a non-neoplastic response to viral replication [1]. In the present paper, a moderate splenomegaly was seen in the affected birds.
In Marek’s disease the majority of transformed T cells express MHC-II and CD4, although CD4−CD8+, CD4+CD8+, and CD4−CD8− T cells also can be transformed by MDV [15]. In addition, the neoplastically transformed cells in MD lymphomas may express high levels of CD25 and CD30 [16] and resemble Treg cells [17]. Immunohistochemical findings of the present study showed that the majority of T cells expressed high levels of CD4-CD8+ especially in the liver. Moreover, the immunohistochemistry for CD30 was positive particularly in the spleen. Probably, these results in this part refer to the virus strain and also species of the affected bird.
In the present paper, it was not possible to identify the source of infection with certainty. The other birds (all of the backyard chickens and the other peafowls) showed no clinical signs. Although, MDV is highly cell-associated but readily transmitted and its virulence varies and evolves. Transmission of MDV from chickens to quail has been reported [1]. MD can be induced in quail by experimental inoculation or contact exposure to MDV strains of both chicken and quail origin [18]. Recently, a case of MD was reported in white-fronted geese (Anser albifrons) [9] which were stated that these species could be considered a reservoir for other avian species [10]. In an older literature, Pradhan et al. [4] already described the occurrence of Marek’s disease in quail located at the same farm where there was a problem of recurrent Marek’s disease among chickens.
To our knowledge, this is the first report of Marek’s disease in green peafowl which infected naturally. In conclusion, we can state that green peafowl are indeed susceptible to Marek’s disease viruses. However, more complimentary studies are required on MDV in this game bird [19,20].
Acknowledgement
The authors are grateful to the Faculty of Veterinary Medicine, University Tabriz, Tabriz, Iran for the financial support.
References
- Schat KA, Nair V (2013) Neoplastic diseases; Marek’s Disease. In: Glisson LR, Dougald MC, et al. (Eds.) Diseases of poultry. (13th edn), John Wiley and Sons Inc., Hoboken, NJ, pp. 515-539.
- Braoudaki M, Tzortzatou-Stathopoulou F (2011) Tumorigenesis related to retroviral infections. J Infect Dev Ctries 5(11): 751-758.
- King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (2011) Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, USA.
- Pradhan HK, Mohanty GC, Mukit A (1985) Marek’s disease in Japanese quails (Coturnix coturnix japonica): a study of natural cases. Avian Dis 29(3): 575-582.
- Pennycott TW, Duncan G, Venugopal K (2003) Marek’s disease, candidiasis and megabacteriosis in a flock of chickens (Gallus gallus domesticus) and Japanese quail (Coturnix japonica). Vet Rec 153(10): 293-297.
- Davidson I, Malkinson M, Weisman Y (2002) Marek’s disease in turkeys. I. A seven-year survey of commercial flocks and experimental infection using two field isolates. Avian Dis 46(2): 314-321.
- Blake-Dyke C, Baigent S (2013) Marek’s disease in commercial turkey flocks. Vet Record 173(15): 376.
- Lesnik F, Pauer T, Vrtiak OJ, Danihel M, Gdovinova A, et al. (1981) Transmission of Marek’s disease to wild feathered game. Vet Med (praha) 26(10): 623-630.
- Murata S, Chang KS, Yamamoto Y, Okada T, Lee SI, et al. (2007) Detection of the virulent Marek’s disease virus genome from feather tips of wild geese in Japan and the far East region of Russia. Arch of Virol 152(8): 1523-1526.
- Murata S, Hayashi Y, Kato A, Isezaki M, Takasaki S, et al. (2012) Surveillance of Marek’s disease virus in migratory and sedentary birds in Hokkaido, Japan. Vet J 192(3): 538-540.
- Campos PF, Gilbert TM (2012) DNA Extraction from Formalin-Fixed Material. Methods Mol Biol 840: 81-85.
- Cao W, Mays J, Dunn J, Fulton R, Silva R, et al. (2013) Use of Polymerase Chain Reaction in Detection of Marek’s Disease and Reticuloendotheliosis Viruses in Formalin-Fixed, Paraffin-Embedded Tumorous Tissues. Avian Dis 57(4): 785-789.
- Burgess SC, Basaran BH, Davison TF (2001) Resistance to Marek’s disease herpesvirus-induced lymphoma is multiphasic and dependent on host genotype. Vet Pathol 38(2): 129-142.
- Coudert F, Vuillaume A, Wyers M, Chaussé AM (1997) Marek’s disease in turkeys. World Poultry 28-29.
- Schat KA, Chen CL, Calnek BW, Char D (1991) Transformation of T-lymphocyte subsets by Marek’s disease herpesvirus. J Virol 65(3): 1408-1413.
- Burgess SC, Davison TF (2002) Identification of the neoplastically transformed cells in Marek’s disease herpesvirusinduced lymphomas: recognition by the monoclonal antibody AV37. J Virol 76(14): 7276- 7292.
- Shack LA, Buza JJ, Burgess SC (2008) The neoplastically transformed (CD30hi) Marek’s disease lymphoma cell phenotype most closely resembles T-regulatory cells. Cancer Immunol Immunother 57(8): 1253-1262.
- Crucillo KL, Schat KA, Schukken YH, Brown AE, Wakenell P S (2010) Pathogenicity of a quail (Coturnix coturnix japonica) derived Marek’s disease virus rescued from the QT35 cell line. Avian Dis 54(1): 126- 130.
- Burgess SC (2004) Marek’s disease lymphomas. In: Davison F, Nair V (Eds.) Marek’s Disease, an Evolving Problem. Elsevier Academic Press, London, UK, pp. 98-111.
- Gimeno IM (2008) Marek’s disease vaccines: a solution for today but a worry for tomorrow? Vaccine 26 (3): C31-C41.
© 2018 Kalantar M. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






