- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Pilot Study on the Use of Adaptive Conjoint Analysis to Assess Risk Factors for Highly Pathogenic Avian Influenza out breaks on Commercial Poultry Holdings of Pakistan
Tariq Abbas
Department of Animal Sciences, University College of Veterinary & Animal Sciences, Pakistan
*Corresponding author:Tariq Abbas, Department of Animal Sciences, University College of Veterinary & Animal Sciences, Bahawalpur , Pakistan
Submission: February 27, 2018; Published: June 12, 2018

ISSN: 2576-9162 Volume4 Issue1
Abstract
This study used Adaptive Conjoint Analysis (ACA) to elicit opinion of veterinarians on risk factors for secondary out breaks of highly pathogenic avian influenza on open-sided chicken farms in Pakistan. A total of 21 risk factors (equivalent to attributes) were evaluated. Web-based ACA interview was administered to thirty-three local veterinarians. The response rate was 39%. Risk factors with highest mean relative importance were: short buffer distance between farms, entry of wild birds into poultry sheds visits of intermediaries and service providers, and sharing high- risk equipment. The paper provides a review of application of ACA as a tool for the elicitation of expert opinion in veterinary epidemiology.
keywords Conjoint analysis; Risk factors; Avian Influenza ;Secondary out breaks; Poultry ; Pakistan
Introduction
For many developing countries, little is known about the relative importance of the risk factors determining introduction, spread and maintenance of Highly Pathogenic Avian Influenza (HPAI) in commercial poultry production units. Quantification of those risk factors through classical epidemiological studies is often difficult for several reasons including data protection, logistics, poor record keeping by farmers, lack of cooperation, inability to control confounders under field situations and potential selection and misclassification bias. In this context, the systematic collection and analysis of opinions and experiences of indigenous experts may be highly valuable to fill the knowledge gaps. Expert opinion has been used to get insight into the epidemiology of various epidemic diseases of livestock [1]. Adaptive Conjoint Analysis (ACA) is one of the techniques available for elicitation of expert opinion.
At first, it was used for marketing research but later applied in a variety of fields like nuclear power industry, engineering, human medicine and to some extent also in veterinary medicine. Conjoint analysis has been used to evaluate the comparative risk and relevance of disease control options [2-12]. The survey technique has some advantages over traditional paper-based or personal interviews. First, ACA is administered via computer. This minimizes interviewer bias and facilitates data collection and management. The computer interface provides respondents a greater degree of anonymity [13] and prevents socio-psychological processes that influence a person’s opinion in a group situation [2].
The data may be collected over the internet which further adds speed, ease, economy in the survey process. Second, ACA focuses on the attributes that are most relevant to the respondent and avoids information overload by focusing on just a few attributes at a time. Moreover, its interactive format captures and holds the participants’ attention in a more powerful way. Thirdly, immediately upon the completion of the interview, the results are available for discussion and analysis. It is also possible to detect and exclude respondents with inconsistent answers [14]. To the best of my knowledge, the technique was never applied in the context of animal diseases in any developing country. This paper describes findings of an ACA study conducted in Pakistan. The main research question addressed was “which risk factors may be important in determining the incidence of HPAI on open house commercial (broiler and layer) chicken farms if HPAI virus enters and establishes itself in Pakistan?”
Materials and methods
The conjoint model is a multi-attribute model, which assumes that consumers purchase products (e.g. an apple) based on the characteristics, or attributes, of the product (e.g. flavor), and that each attribute may have two or more levels (e.g. sweet, tart).
The individual’s utility for a product concept can be expressed in a simple way as the sum of the utilities of its attributes [15]. An epidemic in an animal population also represents a multi-attribute phenomenon. Multiple risk factors “attributes” may have a variable impact in determining the incidence of any disease [2,4]. For example, the type of husbandry can be a risk factor for introduction of virus into a poultry holding [16]. In this example, the attribute “type of husbandry” has three levels, i.e. all-in-all-out (all birds enter together and leave together), all-in, gradual-out (all birds enter together but leave in separate batches over a period of time) and non-specific production system (new birds are introduced into the exiting flocks during production cycle). Bird collectors enter the farm once and at the end of production, therefore all-in-allout husbandry is the preferred method to reduce the likelihood of infection from live bird markets.
A total of 21 risk factors were included in this survey and are given in (Table 1). The list of risk factors was created based on available literature and personal communication with local poultry consultants. At the farm level, the source of virus may be related to the area (i.e. location), pests, people, organic and inorganic items, therefore the risk factors were divided into four categories. Each risk factor was assigned two mutually exclusive levels named as level 1 and level 2 indicating its presence in two extreme scenarios, e.g. “location of farm close to a live bird market” versus “location of farm away from live bird market.” For the area-related risk factors, minimum distance standards were obtained from the literature [17,18].
Table 1: List of potential risk factors included as attributes in the adaptive conjoint analysis survey, and their corresponding sources
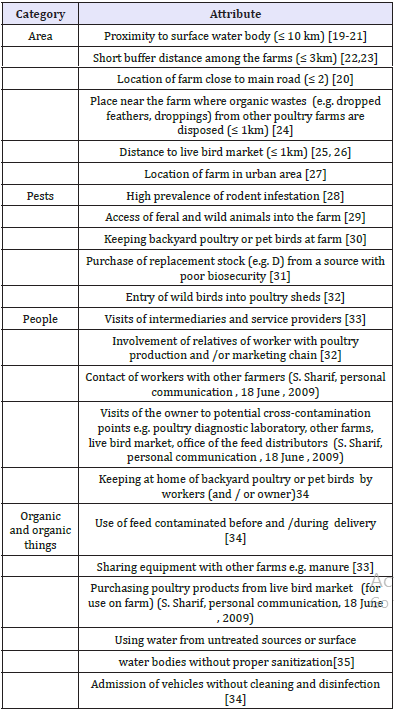
Figure 1: Goat showing the oedematous udder.
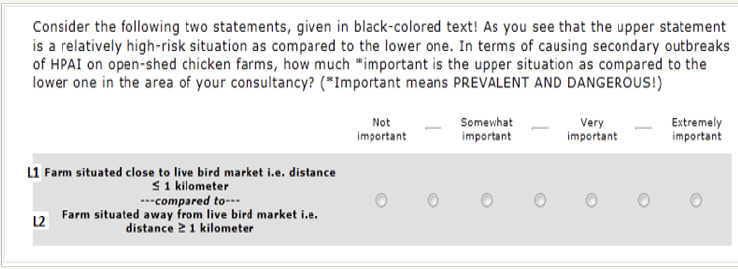
A separate ACA questionnaire was created for each category using ACA/web system version 6.4 (Saw tooth Software, Inc, Sequim, USA). Each questionnaire contained three sets of questions called ranking, paired-comparison and calibration tasks. I did not use the software option “rating task” as the hierarchy of the levels was already known. Number, scale and format of the questions were set according to the instructions given in the documentation of the software. Ranking questions were placed first in the interview and their intent was to assign a score to each risk factor on a sevenpoint Likert scale. (Figure 1) illustrates a prototype ACA ranking question. A single question was asked for each attribute in the response of which the respondents had to compare high risk level (L1) with low risk level (L2) on the basis of its prevalence and ability to cause an outbreak [19-30].
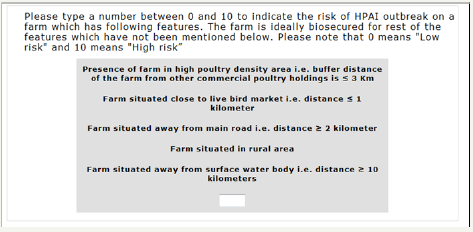
The ranking questions were followed by a series of customized paired-comparison questions (conjoint task). In each paired question, the respondents had to trade-off between combinations of levels from two different risk factors as shown in (Figure 2). ACA is interactive in that it uses the information obtained from each new paired comparison to update utility estimates and to select the next pair of options. Utility measures become more precise as the interview proceeds. The software continues presenting the subject with paired comparisons until enough data have been collected to estimate utilities for each level of each attribute. Mathematical details of these calculations are available at and have been summarized in appendix C. The third and last type of questions asked in the ACA interview was calibration questions. The purpose of the questions was to determine the correlation coefficient in order to assess the level of consistency in the responses. A screen shot of ACA calibration question is given in (Figure 3). In each question, the respondents had to type a number between 0 and 10 (inclusive) to indicate the risk of HPAI out break on a farm with a set of features.
Figure 2: ACA paired comparison question. Combinations of levels from two different attributes are presented side by side. The software automatically selects those on the basis of similarities in utility (risk) score. The respondent has to trade off which combination is relatively more important.
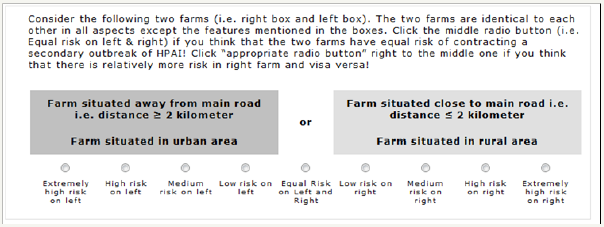
Figure 3: ACA calibration question. Each question contains levels of up to 5 attributes. On a numeric scale from 0-10, the respondent has to give the combined importance of the combination of levels.” 0 “mean low where as 10 means “extremely high risk.
The respondents for this ACA survey were local veterinarians from Pakistan with at least five years experience in control and prevention of poultry diseases. An a priori list of potential respondents was not available. University teachers, field veterinarians from public and private sectors and animal health research workers were consulted to compile a list of 33 potential respondents for this survey. Most of the respondents were contacted directly. In a face-to-face discussion, the respondents were informed about the purpose of the survey and made familiar with the format of ACA questions. This was followed by an email invitation which a link to the questionnaires. The answers to the questions were analyzed by Ordinary Linear Squares (OLS) regression using the ACA Sawtooth software. For each section, the respondents with correlation coefficients equal to or less than 0.8 were excluded from the analysis [31-35].
Results
Among the various sections of the interview, the response rate ranged between 24% and 39%. In total, 13 respondents participated in this survey. The respondents were university teachers, animal health researches, public sector and private veterinarians. Since the number of respondents in each category was quite low, I did not stratify them in the analysis. The median experience of the respondents was 20 years. The median time to complete various sections ranged from 10 to 17 minutes. Three respondents from two different sections (animals, organic and inorganic items) had to be excluded for low level of consistency in their answers. Overall, the level of consistency among the respondents was more than 90%.
(Table 2) shows the relative importance of the risk factors ranked as first, second and third in each risk category. Risk factors with the highest mean relative importance were: short buffer distance among the farms (23.9% ± 10.6%), entry of wild birds into poultry sheds (21.9 % ± 4.8%), visits of intermediaries and service providers (21.2% ±7.1%), and sharing equipment with other farms (38.7 ± 7.2). The analysis of the survey results also revealed differences of opinion among the respondents as indicated by standard deviation. The risk factors showing the highest standard deviation in each category were
Table 2:Mean relative importance of attributes ranked as first, second and third within each risk category
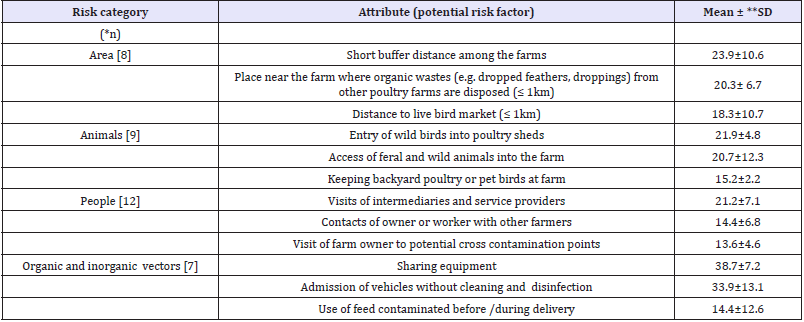
*n= Number of interviews included in analysis, **Relative importance was calculated based on the difference between risk estimates of L1 and L2 of each attribute for each expert, ***SD = Standard deviation As risk factors within each risk category were weighted with respect to each other and independent of those belonging to other categories, the relative importance of a risk factor falling into one category cannot not be compared with that of risk factor belonging to any other risk category.
(i) presence of farm close to main road [± 11.5]
(ii) access of feral and wild animals into farm premises [± 12.6]
(iii) involvement of relatives of worker with poultry production and /or marketing chain [± 9.7] and
(iv) use of feed contaminated before /or during delivery [± 17.3] .
Discussion
The findings of the survey appeared plausible and all the respondents showed a high level of consistency in their answers. For some risk factors, however, I observed high standard deviation which may be due to the small sample size, uncertainties associated with the disease or tendency of the respondents to select middle or end choices of Likert scale. Between 2003 and 2004, HPAI H7N3 caused serious losses to the poultry industry in Pakistan. Exaggerated messages in the media created havoc and shunted the public to non-poultry protein sources. HPAI is therefore a sensitive issue and still a matter of great concern to the government, industry and the community. This was one of the reasons for the reluctance of the respondents to participate in this survey. Another possible reason for the poor response rate appears to be lack of motivation. This might partially be overcome by providing incentives to the respondents or by collecting data during a workshop.
As in all expert elicitation methods, the selection of appropriate experts to participate is vital. Selection of inappropriate, incapable or misrepresentative experts will compromise the process and therefore the opinion elicited [36]. Previously, experts have been selected for participation in expert elicitation procedures based broadly on their experience in the field of interest and professional criteria such as education, publication record and membership of professional societies [37]. Under the conditions prevailing in Pakistan, feasible criteria for selection of expert panel may be experience in the field, willingness to participate in a survey and qualification. To get meaningful results, the knowledge of the veterinarians should be updated about the risk factors being considered. Inserting hyperlinks of the relevant publications in the questionnaire may be helpful in this regard.
The limitations of this survey are inherent to those of small pilot projects and include small sample size and limited generalizability. Another limitation of this survey was the fact that I divided the attributes into categories. As the risk factors within each risk category were weighted with respect to each other and independent of those belonging to other categories, the relative importance of a risk factor falling into one category could not be compared with that of risk factor belonging to any other risk category. In future surveys using ACA, all the risk factors should therefore be considered together. In trade-off questions, combinations of levels from two or more attributes are presented side-by-side on the display of the computer. Ideally and technically, the respondents should consider the levels conjointly. The rank order of the risk factors may be distorted if the respondents subconsciously ignore some levels in decision making (B .McEvan, personal communication, June 6, 2009).
Due to its computer interface, web-based implementation, and questionnaire format, ACA appears to be an attractive alternative to paper-and-pencil techniques for elicitation of expert opinion, however further research is required to prove its feasibility and validity. This can be accomplished by repeating ACA questionnairebased interviews twice on consistent respondents and by calculating Lin’s concordance correlation coefficient [38,39]. In general, expert opinions may have a high degree of uncertainty and be subjected to reporting bias depending upon the political, economic and social implications of the disease under consideration. Experts cannot provide accurate information on the actual impact of a risk factor on the incidence of any disease partially due to spatio-temporal instability of the risk factors; however, an unbiased expert opinion elicited may improve policy making in the absence of data of optimum quantity and quality. Expert opinion-based risk modeling using accurate methods may provide a mechanism of sharing experiences among HPAI-endemic countries and those, which are at-risk or naïve for the disease, without a breach in data privacy.
References
- Garabed RB, Perez AM, Johnson WO, Thurmond MC (2009) Use of expert opinion for animal disease decisions: an example of foot-and-mouth disease status designation. Prev Vet Med 929(1-2): 20-30.
- Staerk KDC, Hosrt HS, Morris RS, Teuffert J (1997) Elicitation of expert knowledge on risk factors for classical swine fever transmission in switzerland. Epidemiol. sante anim 31-32.
- van Schaik G, Dijkhuizen AA, Huirne RB, Benedictus G (1998) Introduction of BHV1 on dairy farms. Risk assessment by cattle farmers and veterinarians. Tijdschr Diergeneeskd 123(6):180-183.
- Horst HS (1999) Risk increase and economic consequences of the introduction of contagious animal diseases in the Netherlands. Tijdschr Diergeneeskd 124(4):111-115.
- Fels-Klerx HJ, Horst HS, Dijkhuizen AA (2000) Risk factors for bovine respiratory disease in dairy youngstock in The Netherlands: the perception of experts. Livestock Production Science 66(1): 35-46.
- Nissen B, joachim krieter (2003) Relative importance of risk factors concerning introduction and spread of classical swine fever and foot and mouth disease in Germany. University of veterinary sciences, Doctoral thesis , Hannover , Germany.
- Sorensen JT, Ostergaard S, Houe H, Hindhede J (2002) Expert opinions of strategies for milk fever control. Prev Vet Med 55(1): 69-78.
- Peddie S, Goddard P, Stott A (2003) Footrot control and sheep welfare : applying adaptive conjoint analysis to elicit stakeholder opinion. In: Goddard P (Eds.) Scottish Agriculture College, Aberdeen , Scotland pp. 24-25.
- Milne CE, Stott AW, Gunn GJ (2005) Using experiential knowledge to evaluate risk factors for Johne’s disease. In: Mellor DJ, Russell AM, Wood JLN (Eds.) Nairn , Scotland, UK.
- Valeeva NI, Meuwissen MP, Lansink AG, Huirne RB (2005) Improving food safety within the dairy chain: an application of conjoint analysis. J Dairy Sci 88(4): 1601-1612.
- Cross P, Williams P, Edwards-Jones G (2009) Differences in the perceptions of farmers and veterinary surgeons about the efficacy of mitigation strategies for controlling bluetongue. Vet Rec 165(14):397- 403.
- Huijps K, Hogeveen H, Lam TJ, Huirne RB (2009) Preferences of cost factors for mastitis management among Dutch dairy farmers using adaptive conjoint analysis. Prev Vet Med 92(4): 351-359.
- Philips CJ, Wojciechowska J, Meng J, Cross N (2009) Perceptions of the importance of different welfare issue in livestock production. Animal 3(8): 1152-1166.
- van Schaik G, Dijkhuizen AA, Huirne RB, Benedictus G (1998) Adaptive conjoint analysis to determine perceived risk factors of farmers, veterinarians and AI technicians for introduction of BHV1 to dairy farms. Prev Vet Med 37(1-4): 101-112.
- Churchill GA, Iacobucci D (1999) Marketing research : Methodologcal foundations. (8th edn). Dryden Press , Fort Worth, TX, USA.
- Toribio JA, Eagles D, Cogger N (2010) Visitor entry to sector 3 poultry farms in Bali and South Sulawesi, Indonesia -Qualitative risk assessment for introduction of HPAI. University of Sydney, Australia pp.28-29
- Ahsan ul Haq (2007) Breeder strategies for disease control.
- Anonymous (2007) Biosecurity guideline for the commercial poultry industry in Bangladesh. Department of Livestock Services (DLS), Bangladesh, Bhuapur, Tangail pp. 27.
- Cecchi G, Ilemobade A, Le Brun Y, Hogerwerf L, Slingenbergh J (2008) Agro-ecological features of the introduction and spread of the highly pathogenic avian influenza (HPAI) H5N1 in northern Nigeria. Geospat Health 3(1):7-16.
- Fang LQ, de Vlas SJ, Liang S, Looman CW, Gong P, et al. (2008) Environmental factors contributing to the spread of H5N1 avian influenza in mainland China. PLoS One 3(5):e2268.
- Gilbert M, Xiao X, Pfeiffer DU, Epprecht M, Boles S, et al. (2008) Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc Natl Acad Sci USA 105(12): 4769-4774.
- Elbers AR, Fabri TH, de Vries TS, de Wit JJ, Pijpers A, et al. (2004) The highly pathogenic avian influenza A (H7N7) virus epidemic in The Netherlands in 2003--lessons learned from the first five outbreaks. Avian Dis 48(3): 691-705.
- Marangon S, Capua I, Pozza G, Santucci U (2004) Field experiences in the control of avian influenza outbreaks in densely populated poultry areas. Dev Biol (Basel) 119:155-164.
- Yamamoto Y, Nakamura K, Okamatsu M, Miyazaki A, Yamada M, et al. (2008) Detecting avian influenza virus (H5N1) in domestic duck feathers. Emerg Infect Dis 14(10):1671-1672.
- Bulaga LL, Garber L, Senne DA, Myers TJ, Good R, et al. (2003) Epidemiologic and surveillance studies on avian influenza in live-bird markets in New York and New Jersey, 2001. Avian Dis 47(3suppl): 996- 1001.
- Choi YK, Seo SH, Kim JA, Webby RJ, Webster RG (2005) Avian influenza viruses in Korean live poultry markets and their pathogenic potential. Virology 332(2): 529-537.
- Pfeiffer DU, Minh PQ, Martin V, Epprecht M, Otte MJ (2007) An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. Vet J 174(2): 302-309.
- http://www.promedmail.org/pls/otn/f?p=2400:1001:163824860538 86882766::NO::F2400_P1001_BACK_PAGE,F2400_P1001_PUB_MAIL_ ID:1000,36355
- Biswas PK, Christensen JP, Ahmed SS, Das A, Rahman MH, et al. (2009) Risk factors for infection with highly pathogenic influenza A virus (H5N1) in commercial chickens in Bangladesh. Emerg Infect Dis 15(12): 1931-1936.
- Terregino C, De Nardi R, Guberti V, Scremin M, Raffini E, et al. (2007) Active surveillance for avian influenza viruses in wild birds and backyard flocks in Northern Italy during 2004 to 2006. Avian Pathol 36(4): 337- 344.
- Kasemsuwan S, Sithisarn P, Poolkhet C, Saguankiat A, T Rakuamsuk, et al. Risk assessment of introduction of HPAI to commercial broiler farms in Thailand. FAVA - OIE Joint Symposium on Emerging Diseases, Bangkok , Thailand 55-56.
- Kung NY, Morris RS, Perkins NR, Sims LD, Ellis TM, et al. (2007) Risk for infection with highly pathogenic influenza A virus (H5N1) in chickens, Hong Kong, 2002. Emerg Infect Dis 13(3):412-418.
- Nishiguchi A, Kobayashi S, Yamamoto T, Ouchi Y, Sugizaki T, et al. (2007) Risk factors for the introduction of avian influenza virus into commercial layer chicken farms during the outbreaks caused by a low-pathogenic H5N2 virus in Japan in 2005. Zoonoses Public Health 54(9-10): 337-343.
- FAO (2008) Bio security for Highly pathogenic avian influenza-Issues and options. Retrieved
- Anjum AD (2007) Possible role of migratory birds for introduction avain influenza in Pakistan
- Webler T, Levine D, Rakel H, Renn O (1991) A novel approach to reducing uncertainty : The group Delphi. Technological Forecasting and Social Change 39(3): 253-263.
- Gallagher E (2005) Studies in quantitative risk assessment: badger-tocattle transmission of Mycobacterium Bovis. PhD thesis , Department of Veterinary Clinical Sciences. Royal Veterinary College, London 250.
- Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45(1): 255-268.
- Cunningham SJ, Hunt NP, Feinmann C (1996) Perceptions of outcome following orthognathic surgery. Br J Oral Maxillofac Surg 34(3): 210-213.
© 2018 Tariq Abbas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






