- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Beneficial Effects of Green Tea Catechin on Veterinary Sciences and Bacterial Infections
Takahiro Iwaya Takahiro Iwaya1, Emiko Isogai1*, Ami Ide1, Takuya Nishimura1, Akiko Saito1 and Lanlan Bai1*
1Department of Animal Microbiology, Tohoku University, Japan
2Department of Engineering, Osaka Electro-Communication University, Japan
*Corresponding author: Emiko Isogai, Laboratory of Animal Microbiology, Graduate School of Agricultural Science, Tohoku University, Sendai, Miyagi 980-0845, Japan
Submission: September 19, 2017;Published: December 07, 2017

ISSN: 2576-9162 Volume2 Issue2
Abstract
The anti-bacterial effects of green tea catechins (GTCs) have been extensively studied. (-)-Epigallocatechin-3-O-gallate (EGCG), the most abundant in GTCs, has shown the highest anti-microbial effect among GTCs. This review focuses on anti-bacterial effects and veterinary use of GTCs and EGCG. EGCG shows inhibition of bacterial growth and bio-film formation, and bactericidal effect, resulting from stress response and damage of cell membrane. In veterinary use, feed addition of EGCG and TE brought health improvement to livestock.
Keywords: Green tea catechins; Anti-bacterial effects; Bio-film
Abbreviations: GTCs: Green Tea Catechins; EGCG: (-)-Epigallocatechin-3-O-Gallate; ECG: (-)-Epicatechin Gallate; EGC: (-)-Epigallocatechin; EC: (-)-Epicatechin; MIC: Minimum Inhibitory Concentration; MBC: Minimum Bactericidal Concentration; CFU: Colony Forming Unit; MRSA: Methicillin- Resistant Staphylococcus Aureus; EHEC: Enterohemorrhagic E. Coli; EC35G: (-)-Epicatechin-3,5-O-Digallate; TE: Tea Extract
Introduction
Figure 1: The representative efficacy of tea catechin and anti-bacterial effect of EGCG.
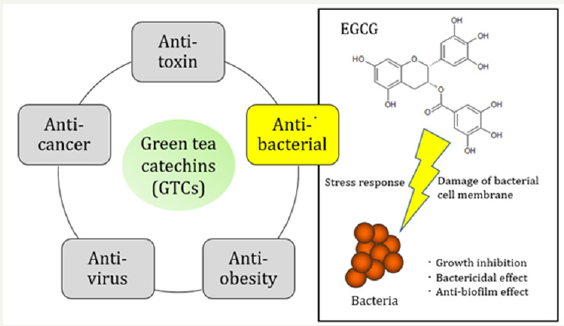
Tea from the leaves and buds of the plant Camellia sinensis is one of the most popular beverages. Green, oolong, and black tea are all obtained from the leaves through full non-fermentation, semi-fermentation, and fermentation, respectively [1,2]. Green tea has beneficial effects against various diseases such as cancer and others [3,4]. Figure 1 shows the representative efficacy of tea catechin. Recently, antimicrobial resistance is an emerging problem worldwide. Antimicrobial usage in animal production is thought to be a contributing factor. A post-antibiotic era is instead a very real possibility for the 21st Century. Green tea extracts and the components including synthetic new poly-phenol could be useful for disease prevention and treatment in veterinary medicine.
Green tea, tea extracts and poly-phenol compounds such as (-)-Epigallocatechin-3-O-gallate (EGCG) show growth inhibition of both Gram-positive and Gram-negative bacteria in-vitro and in-vivo. Green tea catechins (GTCs) acts on microbes but also on enzymes and toxins indicating the compounds deactivate proteins. Infectious diseases are targeted in the study of GTCs [5]. EGCG has shown the highest biological activity among GTCs in most of the studies. This review will discuss briefly the anti-bacterial effects of GTCs and EGCG.
Green Tea Composition
Green tea contains several poly-phenolic compounds, including flavan and flavanol derivatives. Catechins are the most frequent and abundant poly-phenolic compounds [6]. The significant GTCs include EGCG and (-)-epicatechin gallate (ECG), which are produced by the esterification of (-)-epigallocatechin (EGC) and (-)-epicatechins (EC) with gallic acid. EGCG is the most abundant, accounting for 50% [6] to 65% [7] of total catechins.
Anti-Bacterial Effects
Anti-bacterial effects of tea and GTCs have been demonstrated against Gram-positive and Gram-negative bacteria including Staphylococcus aureus, Vibrio cholerae, Escherichia coli, Shigella spp., Salmonella spp., Bacillus spp., Klebsiella spp. and Pseudomonas aeruginosa [8]. Tea extracts were also bactericidal to staphylococci and Yersinia enterocolitica [9]. EGCG inhibits the growth of S. aureus and Escherichia coli [10]. EGCG at the concentrations between 78 and 625μg/mL inhibited multi-drug resistant Acinetobacter baumannii [11]. Various bacteria isolated from canine oral cavity were sensitive to the polyphenolic compounds mix and EGCG [12]. Minimum inhibitory concentration (MIC) ranges were 0.1- 0.8mg/mL for poly-phenolic compounds and 0.0125-0.1mg/mL for EGCG. It is well-known that green tea is effective for caries control. Streptococcus mutans, which is a causative agent of caries, produces insoluble glucan and organic acids in the process of biofilm (plaque) formation. The growth of S. mutans was inhibited by EGCG, ECG, and EGC (the order of their inhibitory effect: EGCG>ECG>EGC), while EC did not show inhibitory function against S. mutans. The MIC and minimum bactericidal concentration (MBC) values of EGCG against S. mutans were 0.125 and 0.1mg/mL, respectively. Tea and tea ingredients showed not only anti-bacterial but also antifungal and antiviral properties.
Anti-Biofilm Effects
Sudano et al. [13] demonstrated that EGCG brought a decrease of slime production and inhibition of biofilm formation by ocular S. aureus and S. epidermidis isolates [13]. Moreover, the colony forming unit (CFU) of S. mutans obtained from biofilm formed in the presence of EGCG were less than that formed in the control culture. These indicate that EGCG had an inhibitory effect on biofilm formation [12]. It has been reported that in addition to binding to lipid layers and peptidoglycan, EGCG interferes with extracellular polymeric material so-called glycocalyx. GTCs significantly inhibit mixed biofilm formation, and reduce the synthesis of auto inducer 2 which is signaling molecule used in quorum sensing [14]. EGCG can eliminate the bio-film matrix by inducing the σE cell envelope stress response and thereby reducing the expression of CsgD [15].
Interaction of Bacterial Cell Components
Initial experiments suggested that negatively charged EGCG exerts its anti-bactericidal activity by binding to the positively charged lipids of the bacterial cell membrane, causing damage to the lipid layer. Subsequently, the interaction of catechins including EGCG with lipid bilayers has been studied in more detail [16,17]. Results from Zhao et al. [10] indicated that EGCG binds directly or indirectly to the peptidogly can of the bacterial cell wall and inhibits the penicillinase activity, protecting penicillin from inactivation [10].
In-vivo model
Steinmann et al. reviewed that the properties of EGCG in various trials against infectious diseases such as methicillinresistant Staphylococcus aureus (MRSA), Stenotrophomonas maltophila, Helicobacter pylori, E. coli and others [5]. We observed that gnotobiotic mice with GTCs had significantly lower Shiga toxin levels than the untreated control group after Enterohemorrhagic E. coli (EHEC) infection [18]. This report also includes the inhibition of bacterial growth in vivo. The untreated controls developed neurological and systemic symptoms, usually culminating in death, whereas none of the mice received green tea extracts exhibited any clinical symptoms or died. The combination of green tea extract and levofloxacin increased survival rates and reduced damage to target organs in orally EHEC infected gnotobiotic mice [19]. Thus, GTCs and EGCG has significant direct and indirect anti-pathogenic effects against food borne bacteria and other bacteria, including multidrug- resistant strains.
Enhancement of Biological Activity by Chemical Modification
The functional and structural differences between these catechins are attributed to the number of hydroxyl groups on the B-ring and the presence or absence of a galloyl moiety. Some chemical modifications have been performed to enhance the biological activity of catechins. Galloyl modification is one of the method to enhance catechins [20]. Galloyl moiety is important to the activity of catechin, it is known that catechins without galloyl moiety have low anti-microbial activity [21]. It is expected that inducing galloyl moiety to original catechins could enhance antimicrobial activity. We demonstrated anti-microbial activity of (-)-epicatechin 3,5-O-digallate (EC35G) [22], galloyl modified catechin from EC. This catechin is made from EC by inducing two galloyl moieties to position 3,5. EC35G inhibited the growth of MRSA, but EC did not.
Use of Tea Extract (TE) for Food Products
The safety and quality of food products are important. The use of antimicrobial film is one of tools for contaminated pathogenic bacteria. For example, incorporation of TE into chitosan-coated films enhanced their effectiveness against Listeiria monocytogenes [23]. Packaging film using TEs could improve the safety of ham steak during room/refrigerated storage. Edible polymer coting with TE was had a significant effect in reducing the fat oxidation of chicken nuggets [24]. Wagh et al. [25] described that plantderived extracts such as TE can be valuable to the modification of frankfurter formulations for improve oxidative stability [25]. Actually, TE showed strong antioxidant activity [26]. Thus, TE could be useful for the safety from bacterial contamination and quality for inhibition of fat oxidation for meat industry and market.
Application of Catechins for Veterinary Science
Much attention was focused on biological activities of catechins in promotion of animals, because human have long applied catechins as dairy drinking. Several are already being tested for medial application. In veterinary science, TE has been used as supplement of feeding for livestock animals (Table 1) [27-33].
Table 1: The veterinary use of tea extract (TE) and EGCG.
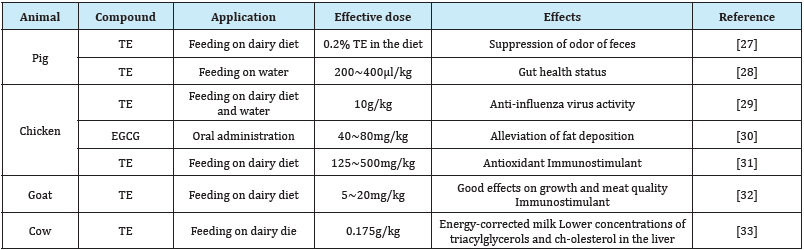
Hara et al. [27] demonstrated that feeding the diet supplemented with 0.2% of tea polyphenols to pigs for 2 weeks, the odor of the feces was reduced [27]. During tea polyphenols administration, the level of lactobacilli was increased whereas bacteroidaceae and clostridia were decreased. Moreover, fecal phenol, p-cresol, and skatole were reduced.
Supplementing broiler chicken diets with TE showed antimicrobial effects including anti-coccidal [34] and anti-influenza activity [29]. As long -term effects in chicken, tea polyphenols improved lipid metabolism and digestive enzymatic activities, and affected inflammatory status [35]. ECG supplementation significantly down-regulated the expression of fatty acid synthesis and β-oxidation/lipolysis genes [30]. They suggested that EGCG could alleviate fat deposition via the improved metabolism. Tea poly-phenols reduced glucocorticoid-induced growth inhibition and oxidative stress in chicken [36]. Tea poly-phenol was also effective to laying hens in the view point of anti-oxidant status, performance of reproduction and egg quality [37].
Mastitis in daily cows is a disease of economic considerable importance [38]. S. aureus is the most common cases of mastitis in dairy cows. In Belgium, MRSA isolates obtained from bovine mastitis cases was first reported in the 1970 [39]. EGCG shows antibacterial effects to not only S. aureus but also MRSA. This suggests that EGCG is useful for treating mastitis caused by MRSA.
Conclusion
GTCs show anti-bacterial effect to Gram-positive and Gramnegative bacteria. This indicates that EGCG is useful for prevention and treatment of infectious diseases in domestic animals. Together with the veterinary use described above, GTCs will be an excellent medicine that brings various efficacies to livestock.
References
- Yang CS, Chen G, Wu Q (2014) Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. J Tradit Complement Med 4(1): 17-23.
- Isogai E, Isogai H, Fujii N, Kimura K, Miura H, et al. (1992) Inhibitory effect of Japanese green tea extracts on growth of canine oral bacteria. Bifidobacteria Microflora 11(2): 53-59.
- Luczaj W, Skrzydlewska E (2005) Antioxidative properties of black tea. Prev Med 40(6): 910-918.
- Chung SY, Xin W, Gang L, Sonia CP (2009) Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer 9(6): 429-439.
- Steinman J, Buer J, Steinmann E (2013) Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol 168(5): 1059-1073.
- Zaveri NT (2006) Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci 78(18): 2073-2080.
- Naglea CG, Ferreiraa D, Zhou YD (2006) Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 67(17): 1849-1855.
- Ech Y (2012) Recent patents on antibacterial, antifungal and antiviral properties of tea. Recent Pat Antiinfect Drug Discov 7(1): 60-65.
- Yam TS, Shah S, Hamilton-Miller JM (1997) Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol Lett 152(1): 169-174.
- Zhao WH, Hu ZQ, Hara Y, Shimamura T (2002) Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob Agents Chemother 46(7): 2266-2268.
- Osterburg A, Gardner J, Hyon SH, Neely A, Babcock G (2009) Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG). Clin Microbiol Infect 15(4): 341-346.
- Bai L, Takagi S, Ando T, Yoneyama H, Ito K, et al. (2017) Antimicrobial activity of tea catechin against canine oral bacteria and the functional mechanisms. J Vet Med Sci 78(9): 1439-1445:
- Sudano RA, Blanco AR, Giuliano F, Rusciano D, Enea V (2004) Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother 48(6): 1968-1973.
- Zhang H, Zho W, Zhang W, Yang A, Liu Y, et al. (2014) Inhibitory effects of citral, cinnamaldehyde, and tea polyphenols on mixed biofilm formation by foodborne Staphylococcus aureus and Salmonella enteritidis. J Food Prt 77(6): 927-933.
- Serra DO, Mika F, Richer AM, Hengge R (2016) The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid fiber assembly and down regulating the biofilm regulator CsgD via the σ(E)- dependent sRNA RybB. Mol Microbiol 101(1): 136-151.
- Kamihira M, Nakazawa H, Kira A, Mizutani Y, Nakamura M, et al. (2008) Interaction of tea catechins with lipid bilayers investigated by a quartzcrystal microbalance analysis. Biosci Biotechnol Biochem 72(5): 1372- 1375.
- Cui Y, Kim SH, Kim H, Yeom J, Ko K, et al. (2012) AFM probing the mechanism of synergistic effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) with cefotaxime against extendedspectrum beta-lactamase (ESBL)-producing Escherichia coli. PLoS One 7(11): e48880.
- Isogai E, Isogai H, Hirose K, Hayashi S, Oguma K (2001) In-vivo synergy between green tea extract and levofloxacin against enterohemorrhagic Escherichia coli. O157 infection. Curr Microbiol 42(4): 248-251.
- Isogai E, Isogai H, Takeshi K, Nishikawa T (1998) Protective effect of Japanese green tea extract on gnotobiotic mice infected with an Escherichia coli. O157:H7 strain. Microbiol Immunol 42(2): 125-128.
- Mizushina Y, Saito A, Horikawa K, Nakajima N, Tanaka A, et al. (2011) Acylated catechin derivatives: inhibitors of DNA polymerase and angiogenesis. Front Biosci (Elite Ed) 3: 1337-1348.
- Umemiya K, Sekine Y, Sato Y, Chiba T, Sonoda M (2016) Effect of tea catechin on folate analysis in green tea by microbiological assay. J Nutr Sci Vitaminol 62(2): 134-138.
- Mori K, Ayano Y, Hamada Y, Hojima T, Tanaka R, et al. (2015) Role of 2,3- cis structure of (-)-epicatechin-3,5-O-digallate in inhibition of HeLa S3 cell proliferation. Nat Prod Chem Res 3: 172.
- Vodnar DC (2012) Inhibition of Listeria monocytogenes ATCC 19115 on ham steak by tea bioactive compounds incorporated into chitosancoated plastic films. Chem Cent J 6(1): 74.
- Kristam P, Eswarapragada NM, Bandi ER, Tumati SR (2016) Evaluation of edible polymer coatings enriched with green tea extract on quality of chicken nuggets. Vet World 9(7): 685-692.
- Wagh RV, Chatli MK, Ruusunen M, Puolanne E, Ertbjerg P (2015) Effect of various phyto-extracts on physico-chemical, color, and oxidative stability of pork frankfurters. Asian-Australas J Anim Sci 28(8): 1178-1186.
- Colon M, Nerin C (2012) Role of catechins in the antioxidant capacity of an active film containing green tea, green coffee, and grapefruit extracts. J Agric Food Chem 60(39): 9842-9849.
- Hara H, Orita N, Hatano S, Ichikawa H, Hara Y, et al. (1995) Effect of tea polyphenols on fecal flora and fecal metabolic products of pigs. J Vet Med Sci 57(1): 45-49.
- Khan SH (2014) The use of green tea (Camellia sinensis) as a phytogenic substance in poultry diets. Onderstepoort J Vet Res 81(1).
- Lee HJ, Lee YN, Youn HN, Lee DH, Kwak JH, et al. (2012) Anti-influenza virus activity of green tea by-products in vitro and efficacy against influenza virus infection in chickens. Poult Sci 91(1): 66-73.
- Li HL, Li ZJ, Wei ZS, Liu T, Zou XZ, et al. (2015) Long-term effects of oral tea polyphenols and Lactobacillus brevis M8 on biochemical parameters, digestive enzymes, and cytokines expression in broilers. J Zhejiang Univ Sci B 16(12): 1019-1026.
- Huang JB, Zhang Y, Zhou YB, Wan XC, Zhang JS (2015) Effects of epigallocatechin gallate on lipid metabolism and its underlying molecular mechanism in broiler chickens. J Anim Physiol Anim Nutr (Berl) 99(4): 719-727.
- Eid YZ, Ohtsuka A, Hayashi K (2003) Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Br Poult Sci F 44(1): 127-132.
- Yuan ZH, Zhang KY, Ding XM, Luo YH, Bai SP, et al. (2016) Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult Sci 95(7): 1709-1717.
- Seegers H, Fourichon C, Beaudeau F (2003) Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res 34(5): 475-491.
- Holmes MA, Zadoks RN (2011) Methicillin resistant S. aureus in human and bovine mastitis. J Mammary Gland Biol Neoplasia 16(4): 373-382.
- Bontempo V, Jiang XR, Cheli F, Lo Verso L, Mantovani G, et al. (2014) Administration of a novel plant extract product via drinking water to post-weaning piglets: effects on performance and gut health. Animal 8(5): 721-730.
- Farahat M, Abdallah F, Abdel-HT, Hernandez-SA (2016) Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response. Br Poult Sci 57(5): 714-722.
- Ahmed ST, Lee JW, Mun HS, Yang CJ (2015) Effects of supplementation with green tea by-products on growth performance, meat quality, blood metabolites and immune cell proliferation in goats. J Anim Physiol Anim Nutr (Berl) 99(6): 1127-1137.
- Winkler A, Gessner DK, Koch C, Romberg FJ, Dusel G, et al. (2015) Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Arch Anim Nutr 69(6): 425-441.
© 2018 Emiko Isogai. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






