- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Trichinellosis: The World Wide Food Originated Zoonotic Disease
Suchit S Pandya*, Hasnani JJ, Patel PV and Hirani ND
Department of Veterinary Parasitology, Veterinary College, India
*Corresponding author: Suchit S Pandya, Department of Veterinary Parasitology, Veterinary College, AAU, Anand-388001, Gujarat, India
Submission: August 12, 2017; Published: November 14, 2017

ISSN : 2576-9162Volume1 Issue5
Introduction
Infection of Trichinella spp. parasite is known as Trichinosis or Trichiniasis. Throughout much of the world, Trichinella spp. have been found to be the causative agents of human trichinellosis, a disease that not only is a public health hazard by affecting human patients but also represents an economic problem in porcine animal production and food safety. Infection by Trichinella spp. has been reported in domestic and wild animals in all the continents, including Antarctica. This parasite builds its own home in the infected muscles by secreting the proteins. The home is a capsule which is composed of a collagenous wall and cellular components [1].
The most important source of human infection worldwide is the domestic pig, but, e.g., in Europe, meats of horses and wild boars have played a significant role during outbreaks within the past three decades. Infection of humans occurs with the ingestion of Trichinella larvae that are encysted in muscle tissue of meat from domestic or wild animals [2].
Due to political and economic changes, recent increases in prevalence and incidence have been observed in many former eastern European countries. Such increases have been related mainly to a reduced efficacy of the veterinary control on susceptible production animals [3].
History
For the first time Trichinella was revealed by Sir James Paget. Sir Rechard Owen wrote up his findings and published them in the transactions of the Zoological Society of London on February 24, 1835 [4]. In 1942 Maplestone and Bhaduri first reported trichinellosis in India [5]. First recognized human case of Trichinella pseudospiralis was reported by Anisworth and his coworkers in 1994 from New Zealand [6]. The sample of diaphragm that Sir Rechard Owen had examined, and that diaphragm had been carefully protected by British Museum and it was destroyed in 1941 by air radiation.
Morphology
It is largest intracellular parasite. Males are smaller than females. Males measures 1.1 to 1.6mm while females are 1.5 to 3.3mm in length. Females are viviparous. Newly shed larva is cylindrical and measures 80-120|i long and 5 to 6 diameter. The body of male is slender and the oesophageal portion is smaller than the posterior part. The hind end of the body bear a pair of lateral flaps on either side of cloacal opening, with two pairs of papillae behind them. In body of female, vulva situated near the middle of the oesophageal region the egg measures by 40 by 30|im and contained fully developed embryo when present in the uterus of the mother (http://www.trichinella.org/) (Figure 1).
Figure 1
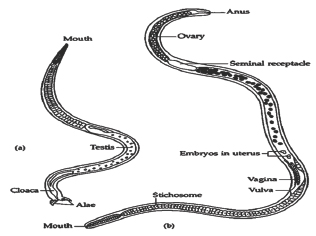

Twelve genotypes of genus Trichinella have been identified, eight of which have been designated as species. Several other species of Trichinella have number of designations only [7].
i. T. spiralis, T1
ii. T. nativa, T2
iii. T. britovi, T3
iv. T. pseudospiralis, T4
v. T. murrelli, T5
vi. T6
vii. T. nelsoni, T7
viii. T8
ix. T9
x. T. papuae, T10
xi. T. zimbabwensis, T11
xii. T12
Epidemiology
Fifty percent infection in domestic animals as well as 47.9% in human is recorded in Europe [8]. During 1986-2009, total 64338 cases were reported in humans with 36 cases of death (www.cdc. gov, 2011). From remote areas of Uttarakhand, 42 humans cases were reported including 11 death (www.cdc.gov, 2012). Former Uttarakhand Chief Minister and BJP National Vice-President Ramesh Pokhriyal Nishank had been affected by the disease (www. dailypioneer.com, 2011) (Table 1 & 2).
Table 1:
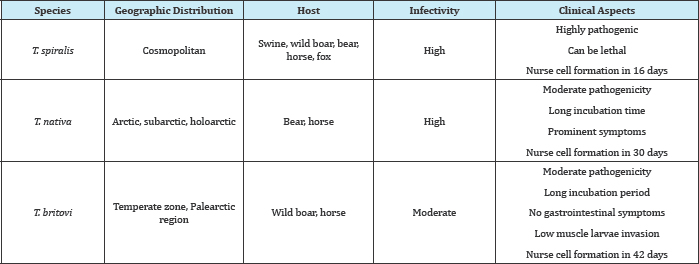
Table 2:

Summary of trichinellosis outbreaks reported from 2008 to at present (Tables 3-5):
Table 3:
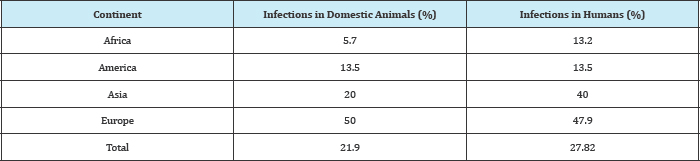
Table 4:
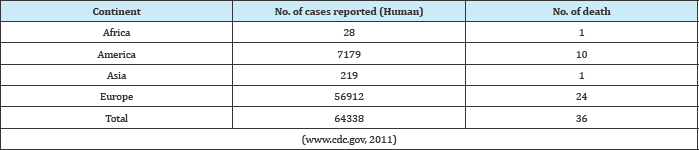
Table 5:

Source: (www.dailypioneer.com, 2011).
i. 28 Jul 2012 Trichinellosis - Argentina (02): (La Pampa) porcine
ii. 12 May 2012 Trichinellosis - Argentina: (NQ, CB)
iii. 23 Mar 2012 Trichinellosis - Chile: (CE)
iv. 23 Nov 2011 Trichinellosis - Chile : (LR)
v. 28 Oct 2011 Trichinellosis - Russia: (AL)
vi. 22 Oct 2011 Trichinellosis - Argentina (02): (CN) swine
vii. 19 Oct 2011 Trichinellosis - India: (UT)
viii. 21 Aug 2011 Trichinellosis - Argentina: (CB)
ix. 24 Jul 2011 Trichinellosis - Latvia: (DV)
x. 06 Mar 2011 Trichinellosis, fatal - Spain: (AR) wild boar meat
xi. 09 Jan 2011 Trichinellosis - Ukraine: (CV), RFI
xii. 06 Nov 2010 Trichinellosis - Argentina (02): (CB)
xiii. 24 Aug 2010 Trichinellosis - Chile: (VD)
xiv. 13 Aug 2010 Trichinellosis - Argentina: (ER)
xv. 05 Jul 2010 Trichinellosis - Mexico: (OA)
xvi. 15 Dec 2009 Trichinellosis - Belarus: (BR) wild boar meat
xvii. 05 Nov 2009 Trichinellosis - Russia (04): (VR) badger meat
xviii. 23 Oct 2009 Trichinellosis - Russia: (KE), bear meat
xix. 07 Oct 2009 Trichinellosis - France ex Canada: (NU)
xx. 27 Sep 2009 Trichinellosis - Lithuania: (VI) wild boar meat
xxi. 16 Jul 2009 Trichinellosis, dog meat, human - Russia: (ZB)
xxii. 21 May 2009 Trichinellosis, warthog ham - France ex Senegal
xxiii. 05 Apr 2009 Trichinellosis - Russia: (KX)
xxiv. 08 Mar 2009 Trichinellosis - China: background
xxv. 07 Mar 2009 Undiagnosed fatal illness - China (02): (YN) trichinellosis
xxvi. 23 Nov 2008 Trichinellosis, porcine - Germany: (Western Pomerania)
xxvii. 23 Nov 2008 Trichinellosis - Russia (06): (Kemerovo Region)
xxviii. 19 Sep 2008 Trichinellosis - Russia (05): (Chukchi Autonomous Region)
xxix. 07 Sep 2008 Trichinellosis - Russia (04): Magadan
xxx. 22 Jul 2008 Trichinellosis, salami - Argentina: (SFE)
xxxi. 06 Jul 2008 Trichinellosis - Russia (03): (Tomsk), bear meat
xxxii. 22 Jun 2008 Trichinellosis - Russia (02): (Zabaykalye, Tomsk)
xxxiii. 23 Feb 2008 Trichinellosis - Russia (Krasnodar)
Transmission
Figure 2
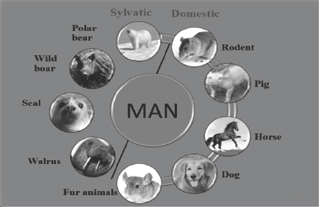
Transmission of Trichinella spp. to the humans can take place from both sylvatic as well as domestic animals. Sylvatic side it is transmitted from polar bear, wild boar, seal, walrus. Domestic side it is transmitted from pig, horse, dog, fur animals and rodents. So seal and walrus comes under marine animals (Figure 2).
Lifecycle
It is divided into two phases, Enteral phase and Parentral phase (Figure 3).
Figure 3
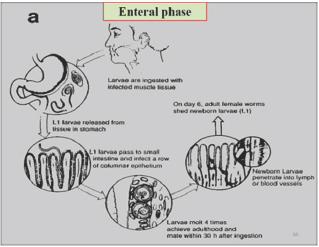
Mode of action
Infection is initiated by ingesting raw or uncooked meat harboring the Nurse cell-larva complex. Larva are released from muscle tissue by digestive enzyme in the stomach and then locate to the upper two third of the small intestine. The outer most cuticular layer becomes partially digested. This enables the parasite to receive environmental cues and then to select the infection site within the small intestine. The immature parasite penetrates the columnar epithelium at the base of the villus. Larvae molts four times in rapid succession over a thirty hour period developing into adults. Copulation occur about 40 hours of infection, after copulation male dies and female penetrate into mucosa of small intestine and some may reach to the lymph space. Here they produce, over a period of several weeks, eggs that hatch inside the uterus of the female. Adult female worms lay the larvae (L1) [9].
Parenteral phase
New born larvae migrate through the body of the host via lymph and blood. New born larvae penetrate out of the blood vessel. 4 days after penetrating muscle cell nurse cell begins to form. 12 days after penetrating muscle cell nurse cell formation almost complete. Mature nurse cell- L1 larva complex is fully developed by 20 days after entering in muscle [9](Figure 4).
Pathogenesis
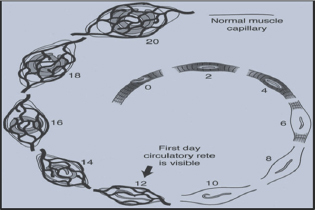
The most important pathogenic effect produced by the larvae is in the muscle is formation of nurse cell. The larvae enters in the striated muscle fibers and cause Loss of muscle proteins and enzymes (e.g. actin, myosin and creatinine kinase). No muscle contractile proteins can be detected beyond Day 8 after the parasite invades the muscle cell. Larva surrounded by a collagen capsule formed from the muscle fiber. There is modulation or re-differentiation in the structure of the muscle cell. It is termed as Nurse Cell. To nourish themselves in the nurse cell, the larvae stimulate angiogenesis leading to formation of a capillary rete around the invaded muscle cell [9] (Figure 5).
Figure 4
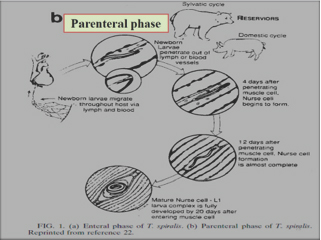
Figure 5
Public health hazard/importance
European commission had given guidelines, Meat containing at least 1 larva per gram is necessary to induce a clinical infection in man. The sudden occurrence of high fever, facial edema and myalgia in a group of persons suggests the presence of Trichinella infection [10]. linical forms of TTichinellosis based on disease severity are severe, moderately severe, benign, abortive, and asymptomatic [2].
Different clinical forms having various incubation periods, more severe form having incubation period generally shorter, severe form having incubation period 1 week, moderately severe form having incubation period 2 weeks, benign and abortive form having incubation period 3-4 weeks [2]. Most common initial symptoms are diarrhea, fewer, myalgia. Myalgia seen in 75% cases. Most common sites are masseter, diaphragm, and intercostal muscles. High percent reported in extremities and neck/shoulder girdle up to the severity, inability to ambulate or perform simple upper extremity or truncal tasks like feeding or sitting [2]. Main clinical signs are periorbital edema, facial edema, edema of bulbar conjunctiva, chemosis, myositis, splinter hemorrhage [2].
Diagnosis
There are two testing methods for the detection of Trichinella infection. One is direct method for detection and second is indirect method for detection [11]. The only recommended procedure for the detection of Trichinella larvae in muscle tissues is digestion assay. The International Commission on Trichinellosis (ICT) recommends this assay, which is documented standards in the European Union (EU), Canada and elsewhere in other countries [12]. Direct method can identify pigs, horses or other animals infected with Trichinella spp. as early as 17 days after exposure. Direct method is most sensitive on fresh samples because the number of larvae that can be recovered from samples, declines unpredictably after prolonged storage, putrefaction and freezing [7]. Muscles are taken from different predilection from different animals. In Pigs muscles are taken from the diaphragm, tongue, masseter muscles. In horses muscles are taken from tongue, masseter muscle. In wild animals predilection sites are unknown but tongue, diaphragm or masseter should be taken [7].
Digestion assay
Take meat sample and trimmed it to remove fat and fascia and make it 100g amount and add 50-100ml of the preheated Digestive solution (water/HCl solution) for a 100g sample. Chop the meat in a blender until it is homogeneous. Sprinkle 10g of pepsin into the homogenate, again add about 200ml of water/HCl solution and blend for about 5 seconds. Transfer the homogenized sample to a 3-litre beaker containing a stir bar. Allow the digestion to proceed for 30 minutes than filter through 177-180μm sieve. Allow the fluid undisturbed for 30 minutes to settle. Remove supernatant and collected sediment quickly transfer into a petridish and examine under stereomicroscope [7].
Polymerase chain reaction
This is the molecular method. It is used to detect the nucleic acid of larvae in the musculature. However, this method is not practical for routine testing of food animals. It is costly method. It is mainly uses for identification of the species or genotype of Trichinella recovered from muscle tissue is useful in understanding the epidemiology of the parasite in animals [13].
Trichinoscopy
This method involves the compression of multiple 2 x 10mm pieces of muscle tissue between two glass plates until they become translucent followed by examination using a microscopic technique. Trichinoscopy is not as sensitive as digestion assay, so it is not recommended by the ICT or EU for the routine examination of carcasses [11] (Table 6).
Table 6:
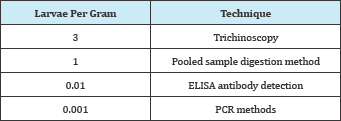
Serological tests
The ELISA is the only immunological assay endorsed by the ICT. It is only approved as an epidemiological surveillance tool to detect anti-Trichinella antibodies in pigs. It is not reliable for the detection of Trichinella infection in other animals. Disadvantage of this method also there. Low rate of false-negative results observed in infected animals. This is primarily due to detectable levels of antibody are not usually present in pigs until 3-5 weeks or more following exposure For this reason, serological methods are not recommended for individual carcass testing [14].
Prevention and Control
Following methods are uses for prevention and control of this disease.
Slaughter testing
After doing slaughter in slaughter house testing of each carcass can be done. Slaughter testing of swine can be done. Slaughter testing of horse can be done. Slaughter testing of game animal meats can be done. After doing testing of carcasses recommended actions taken when a positive test result is obtained. Develop quality assurance systems for digestion testing [15].
Processing methods to control trichinellosis
Cooking to inactivate trichinella: Guidelines set by the United States Department of Agriculture's Code of Federal Regulations (1990) (Appendix C) for treatment of meat to prevent human Trichinellosis. Meat must be cooked up to 71°C. A change in color from pink to grey throughout, and a change in texture such that muscle fibers are easily separated from each other are indicators that meat has been rendered safe to eat [15].
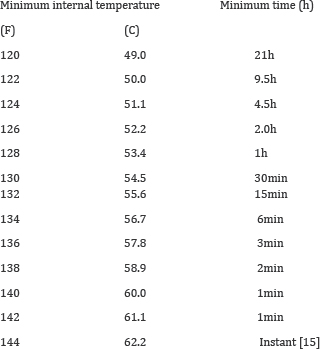
Freezing to inactivate trichinella: Guidelines set by the United States Department of Agriculture's Code of Federal Regulations (Appendix D) are acceptable for treatment of meat to prevent human Trichinellosis. Cuts of meat up to 15cm in thickness are frozen solid (at least -5 °C) for no less than 3 weeks and Cuts of meat up to 69cm in thickness are frozen solid (at least -15°C) for no less than 4 weeks [15]. T. britovi larvae in pork have survived up to 3 weeks at -20 °C, T. spiralis larvae in horse meat frozen at -18 °C (-0.4 °F) can survive up to 4 weeks [1].
Irradiation to inactivate trichinella: ICT considers irradiation, at levels proven to inactivate Trichinella (0.3kGy), to be an acceptable method for rendering meat safe for human consumption [15].
Consumer education: Educate the consumer for various methods of prevention. Cooking to an internal temperature of 71aC (160 °F). Freezing solid (-15 °C or less) for 3 weeks (cuts up to 15cm in thickness). Freezing solid (-15 °C or less) for 4 weeks (cuts up to 69cm in thickness). In areas where freeze-resistant Trichinella are endemic, consumers should be informed that freezing is not recommended. Methods for preparation of meats which are not considered secure include cooking using microwaves, curing, drying, or smoking [15].
On-Farm Control
On the pig farms requirements for Trichinella free pig production. Requirements for Trichinella-free production of horses. Certification of Trichinella-free livestock production, measures of Architectural and environmental barriers can be taken. Control of rodents can be done on the farm. Maintain hygienic condition on farm and good management of feed and feed storage.
Requirements for vaccines
There are no vaccines for Trichinellosis in food animals or game animals [7].
Trichinella organizations and reference laboratories:
International commission on trichinellosis (ICT): The principal functions of the ICT are:
i. The promotion and facilitation of studies relating to all phases of Trichinella infection in animals and humans
ii. Hosting of an International Conference on Trichinellosis every 4 years for presentation of reports on all aspects of Trichinella and trichinellosis. Organizing or participating in national or international congresses, symposia.
International reference laboratories: The OIE has established two reference laboratories for Trichinellosis
i. Centre for Foodborne and Animal Parasitology, Canadian Food Inspection Agency, Saskatoon, Canada
ii. Istituto Superiore di Sanita, Rome, Italy
Treatment
i. Albendazole is given @400mg twice daily for 8 to 14 days. Mebendazole is given @200 to 400mg three times a day for 3 days, followed by @400 to 500mg three times a day for 10 days [16] (Table 7).
Table 7:
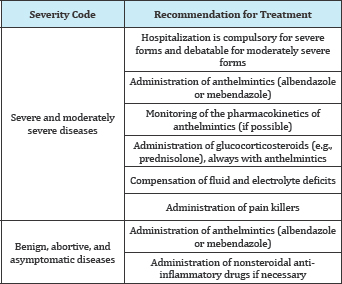
ii. They are contraindicated during pregnancy and not recommended in children aged <2 year [17].
iii. Steroids, e.g., prednisone, administered at a dose of 30mg/ day to 60mg/day for 10 to 15 days for severe symptoms [18-20].
iv. Pyrantel is given in a single dose of 10 to 20mg/kg of body weight, repeated for 2 to 3 days, and may be used by pregnant women and children, but it is active only against worms in the gut, and it has no effect against newborn and muscle larvae [2].
Conclusion
i. Infection of Trichinella is known as Trichinosis or TTichiniasis
ii. Infection by Trichinella spp. has been reported in domestic and wild animals in all the continents, including Antarctica.
iii. For the first time Trichinella was revealed by Sir James Paget.
iv. Twelve genotypes of genus Trichinella have been identified.
v. During 1986 - 2009, total 64338 cases were reported in human with 36 cases of death
vi. During the year 2011 from Uttarakhand 42 human cases were reported including 11 human death.
vii. Trichinella spp. infection in human was documented in 55 (27.8%) countries.
viii. Main clinical signs of Trichinella are facial edema, chemosis, splinter hemorrage, myositis, periorbital edema.
ix. Diagnosis of Trichinellosis can be done by using Digestion assay, PCR, Serological methods, TMchinoscopy.
x. Prevention and control of the disease can be done by Freezing, Cooking, Curing, Irradiation and by Consumer education.
xi. Treatment of the disease can be done by using Albendazole, Mebendazole, Glucocorticosteroides and pyrantel.
Future Prospects
i. Epidemiological studies are required urgently because the infection is likely to be under-diagnosed, and it is necessary to explore the existence of the parasite among wildlife reservoirs, pig, horse meat etc.
ii. There is a need for development of vaccine for Trichinellosis
iii. There is a need to educate the people about the infection of the disease with the help of the extension workers.
iv. Need to establish good laboratories that will perfectly diagnose the disease.
v. Certification program for Trichinella-free farms need to be establish.
References
- Pozio E, Murrell KD (2006) Systematics and epidemiology of Trichinella. Adv Parasitol 63: 367-439.
- Camet JD, Bruschi F (2007) Management and diagnosis of human trichinellosis. AGRIS pp. 37-68.
- Blaga R, Durand B, Antoniu S, Gherman C, Cretu CM, et al. (2007) Dramatic increase in the incidence of human trichinellosis in Romania over the past 25 years: impact of political changes and regional food habits. Am J Trop Med Hyg 76(5): 983-986.
- Owen R (1835) Description of a microscopic entozoon infesting the muscles of a human body. Trans Zool Soc Lond 1(1835): 315-332.
- Niphadkar SM, Pradhan MH, Deshpande VS (1979) Rediscovery of Trichinella spiralis in domestic pigs in India. Curr Sci 48: 372-373.
- Ancelle T (1998) History of trichinellosis outbreaks linked to horse meat consumption 1975-1998. Euro surveill 3(8): 86-89.
- OIE (2012) Trichinellosis. In: Manual of diagnostic tests and vaccines for terrestrial animals (10th edn) Office International des Epizooties. Paris, France.
- Pozio E (2007) World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol 149(1-2): 3-21.
- Capo V, Despommier DD (1996) Clinical aspects of infection with Trichinella spp. Clin Microbiol Rev 9(1): 47-54.
- European Commission (2005) Commission Regulation (EC) No. 2075/2005 of 5 December 2005 laying down specific rules on official controls for Trichinella in meat. Off J European Union 338: 60-82.
- Gajadhar AA, Pozio E, Gamble HR, Nockler K, Maddox-HC, et al. (2009) Trichinella diagnostics and control: mandatory and best practices for ensuring Food safety. Vet Parasitol 159: 197-205.
- Canadian food inspection agency (2010) Meat hygiene manual of procedures, chapter 5, sampling and testing, section 5.5.2.7.6, double separatory funnel.
- Pozio E, La-Rosa G (2003) PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol Biol 216: 299-309.
- Gamble HR, Pozio E, Bruschi F, Nockler K, Kapel CMO, et al. (2004) International Commission on Trichinellosis: Recommendations on the use of serological tests for the detection of Trichinella infection in animals and man. Parasite 11(1): 3-13.
- Gamble HR, Boireau P, Nockler K, Kapel CMO (2007) Prevention of Trichinella infection in the domestic pig. Parasite pp. 99-108.
- Anonymous (2004) Drugs for parasitic infections. Med Lett pp. 1-12.
- Horton J (1993) The use of antiprotozoan and anthelmintic drugs during pregnancy and contraindications. J Infect 26(1): 104-105.
- Shimoni Z, Klein Z, Weiner P, Assous MV, Froom P (2007) The use of prednisolone in the treatment of trichinellosis. Isr Med Assoc J 9(7): 537-539.
- http://www.dailypioneer.com/nation/13827-ten-die-after-consuming- infected-meat-in-pauri.html.
- http://wwwnc.cdc.gov/eid/article/17/12/11-0896_intro.htm.
© 2017 Suchit S Pandya, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






