- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Preliminary Evaluation on the Bio-Activity of Herb and Spice Extracts Against Bovine Mastitis Causing Bacteria
Fabio Mastrogiovanni1, Pier Paolo Danieli1*, Antonio Tiezzi2, Antonino Barone3, Luca Silveri3, Lorenzo Gallipoli1, Silvia Abbruzzese3 and Nicola Lacetera1
1Department of Agricultural and Forestry Sciences (DAFNE), University of Tuscia, Viterbo, Italy
2Department for the Innovation in Biological, Agro-food and Forest systems (DIBAF), University of Tuscia, Viterbo, Italy 3lstituto Zooprofilattico Sperimentale del Lazio e della Toscana "M. Aleandri'', Viterbo, Italy*Corresponding author: Pier Paolo Danieli, Department of Agricultural and Forestry Sciences (DAFNE), University of Tuscia, Via S. Camillo De Lellis, 01100 Viterbo, Italy
Submission: October 04, 2017; Published: November 13, 2017

ISSN : 2576-9162Volume1 Issue4
Abstract
Mastitis cause serious economic losses to dairy farmers, and strategies for their control need continuous implementation. In this study, water and ethanol extracts of leaves of Myrtus communis (MC), Rosmarinus officinalis (RO) and Salvia officinalis (SO) and the spice from the bark of Cinnamomum verum (CV) were in vitro tested for their potential antibacterial activities against common etiological agents of mastitis. The antibacterial activity of plant extracts was tested by the standard paper disk-diffusion method (Kirby-Bauer antibiotic testing). Both CV and MC extracts showed antibacterial activity against B. cereus and S. aureus; the MC extract exerted bacteriostatic effects also to S. uberis. The RO extract showed activity against B. cereus, S. aureus, and S. agalactiae whereas the antibacterial activity of SO was only to B. cereus. In our conditions, except for the CV water extract, extraction in ethanol resulted more effective than that in water suggesting that active substances are preferably extractable from plant materials by organic solvents. However, none of the extracts showed antibacterial activity against E. coli, P. aeruginosa, and S. dysgalactiae.
Keywords: Antibacterial activity; Agar diffusion assay; Bovine mastitis; Plant extracts
Abbreviations: ZOI: Zone of Inhibition; B. cereus: Bacillus cereus; CV: Cinnamomum verum; DMSO: Dimethylsulfoxide; E. coli: Escherichia coli; Et: Ethanol extract; F: Filtered extract; MC: Myrtus communis; nd: Not detectable inhibition zone diameters; OD: Optical density; P. aeruginosa: Pseudomonas aeruginosa; RO: Rosmarinus officinalis; SO: Salvia officinalis; S. aureus: Staphylococcus aureus; SW: Sterile Ultrapure Water; S. agalactiae: Streptococcus agalactiae; S. dysgalactiae: Streptococcus dysgalactiae; S. uberis: Streptococcus uberis; UF: Unfiltered Extract; W: Water Extract ; SEM: Standard Error of the Mean
Introduction
Mastitis remains a major challenge to the worldwide dairy industry despite the widespread implementation of mastitis control strategies [1]. Mastitis etiology can have an infectious or non-infectious origin, though the majority of clinical and sub- clinical cases are mainly of bacterial origin. Overall, a small number of microorganisms are imputable of being the causal agent of bovine mastitis and are routinely classified either as "contagious" (e.g., Staphylococcus aureus) or "environmental" (e.g., Escherichia coli) pathogens; less common causes of mastitis are attributable to Bacillus species, in particular Bacillus cereus may produce an acute, gangrenous mastitis [2]. Antibiotic resistance is a consequence of a massive use of antibiotics as growth promoters or as tools against microbial infections [3] and the uncontrolled application of antibiotics in the past contributed to select resistant bacteria and as a consequence problems are presently occurring in the use of antibiotics for animal and human needs. In this view, a continuing search for new antibiotics is running and a number of plants have been recognized as promising natural sources of antibacterial molecules [4]. Since long time leaf, bark and root extracts are being used for their antibacterial properties and plants already used in traditional medicine, further reinvestigated by the modern bio-medical research, can often result as a good source of new antibiotics. In the framework of a wide research project on plants with potential antibacterial properties and in the perspective of their use in the veterinary practice, we tested some plant extracts on bacteria responsible for bovine mastitis.
Materials and Methods
Plant materials and extraction procedure
Leaves of Myrtus communis (MC), Rosmarinus officinalis (RO), Salvia officinalis (SO) and spice from the bark of Cinnamomum verum (CV) were used for performing in vitro antibacterial tests on the main bacterial strains causing bovine mastitis. Leaf or bark samples were processed by extraction with two different solvents, ethanol (Et) and water (W). Two aliquots (5 g) of leaves were washed with distilled water, dried at room temperature and 20 mL of solvents added. Leaves were homogenated by an Ultra-Turrax T18 basic (IKA®, Germany) until homogeneous mixtures were obtained. For CV bark, the washing and homogenization steps were skipped because samples were directly purchased as a commercial powder (CANNAMELA Srl). Two aliquots of cinnamon powder (5 g) were dispersed in ethanol or water vortexed and homogeneous mixtures obtained. The leaves and bark mixtures were then left in the dark at room temperature for 90 minutes.
Afterwards, the mixtures were centrifuged at 4,000 rpm (Rotofix 32, Hettich®, Germany) for 5 minutes, the debris discarded and subsequently the supernatants were centrifuged at 14,000 rpm (Mikro 120, Hettich®, Germany) for 10 minutes. Pellets were discarded and the supernatants dried at room temperature under a pure nitrogen stream. For testing possible antibacterial activity, the ethanol extracts (Et) were re suspended in 10% dimethylsulfoxide (DMSO) [5] and the water extracts (W) in ultrapure water (ASTM Type I), at a nominal concentrations of 20 mg of dry extracted solids per milliliter. One aliquot of each solution was filtered by using 0.22 |im sterile filters (F) to evaluate if filtration of possible fine, undissolvable, particle materials may lead to different results in comparison with unfiltered counterpart solutions (Uf).
Subsequently, F and Uf solutions were tested on mastitis causing bacteria. For Et extracts a control of 10% DMSO in sterile ultrapure water was included and for W extracts a control of sterile ultrapure water (SW) was included.
Bacterial cultures and antibacterial activity
The bacterial strains causing bovine mastitis [6,7] such as Bacillus cereus ATCC 10876 (B. cereus), Escherichia coli ATCC 35218 (E. coli), Pseudomonas aeruginosa ATCC 10145 (P. aeruginosa), Staphylococcus aureus ATCC 25923 (S. aureus), Streptococcus agalactiae ATCC 27956 (S. agalactiae), Streptococcus dysgalactiae ATCC 43078 (S. dysgalactiae) and Streptococcus uberis ATCC 700407 (S. uberis) were cultured at 37 °C.
B. cereus, E. coli, P. aeruginosa and S. aureus were plated in Mueller Hinton Agar whereas S. agalactiae, S. dysgalactiae and S. uberis were plated in Mueller Hinton Agar with 5% of sheep blood [8]. The antibacterial activity of plant extracts was evaluated by the standard agar disc diffusion method [9]. Each bacterial strain was suspended in SW and measured by a spectrophotometer (Spectronic 21, Milton Roy Company) to obtain an optical density (OD) of 0.125 at 550 nm (0.5 McFarland of turbidity). Aliquots of 100 |il of each suspension were plated in Petri dishes containing agarized medium. For each test seven discs (Blank Antimicrobial Susceptibility Disks, Oxoid™) soaked with 20 |il of the same extract were placed in the plate; two additional discs, one soaked with 20 |il of 10% DMSO or SW (negative control) and the other one presoaked with antibiotic to which the bacterium was known to be sensitive (positive control) were also positioned.
In the E. coli, S. aureus and S. dysgalactiae experiments, presoaked discs contained 10 ng of Gentamicin (Gentamicin Antimicrobial Susceptibility Disks, Oxoid™); in the experiments with B. cereus and P. aeruginosa, presoaked discs contained 5 Hg of Enrofloxacin (Enrofloxacin Antimicrobial Susceptibility Disks, Oxoid™); in the S. uberis experiments, discs containing 10 Hg of Ampicillin (Ampicillin Antimicrobial Susceptibility Disks, Oxoid™) were used; when testing the extract against S. agalactiae, discs containing 30 ng Tetracycline (Tetracycline Antimicrobial Susceptibility Disks, Oxoid™) were used.
The Petri dishes were then incubated for 24h at 37 °C. Antibacterial activity of positive controls was assumed to be 100% and evaluated by measuring the zone of inhibition (ZOI) diameter. In order to calculate the relative inhibiting effect, the ZOI diameter was proportioned to the respective positive control.
Statistical analysis
The relative inhibiting effect was analyzed by full factorial ANOVA (Statistica 10, Stat Soft). The influence of extraction solvent and filtration treatment on the in vitro antibacterial activity of each extract was assessed according to the following model:
yijk = μ + Si + Fj + (S+F)ij + εijk
where y was the relative inhibition response, n the global mean, S the effect of the extraction solvent (W, Et), F the effect of the filtration treatment (Uf, F), S x F the first order interaction and s the error term. Significant differences between means at the 5% level were evaluated using the LSD Fisher test.
Results and Discussion
Table 1: Bacterial strains treated with the reference antibiotics used as positive control and 10% Dimethyl Sulfoxide (DMSO10%) solution or sterile ultrapure water (SW) as negative controls.
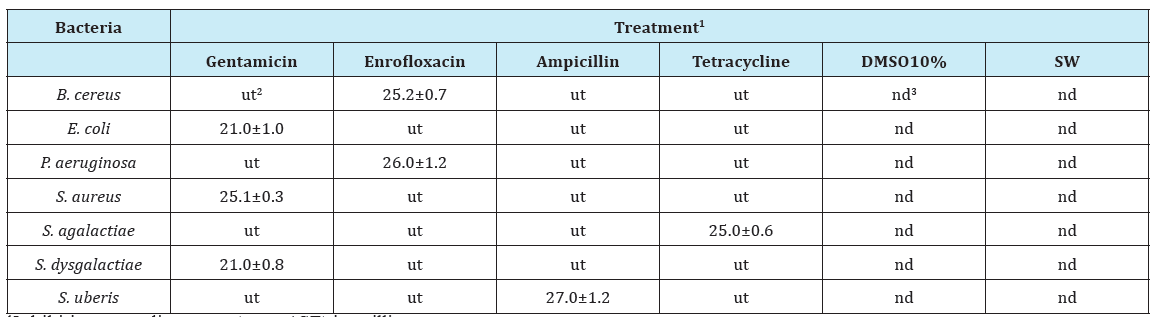
1Inhibition zone diameters (mean±SE) in millimeters;
2Bacterial strains untreated with antibiotics;
3Not detectable inhibition zone diameters.
On sensitive bacterial strains the respective positive controls showed a ZOI ranging from 21 to 27 mm (Table 1). As expected, negative controls (DMSO 10% and SW) did not show any inhibition zone on all bacteria strains. The extracts showed different levels of activity against the bacterial strains used in the study (Table 2). In particular, all extracts showed relative inhibitory activities against B. cereus ranging from 31, 25%, given by the ethanolic unfiltered SO extracts, to 43, 15% obtained with water unfiltered CV extracts.All CV extracts showed a strong inhibitory activity against B. cereus and S. aureus. These results agree with those already reported by Joshi & coworkers [10], although these authors tested only unfiltered ethanol extracts obtaining a ZOI of 10 mm for both strains with a minimum bactericidal concentration of 10 mg/ml. In our experiments the CV water extract provided interesting results with about 10 mm of ZOI for B. cereus and 9 mm for S. aureus with a concentration of 20 mg/ml.
Table 2: Results of the ANOVA on the relative Inhibition response of plant extracts on mastitis causing bacteria.

S: Solvent; F: Filtration; W: Water Extract; ET: Ethanol Extract; UF: Unfiltered Extract; F: Filtered Extract; CV: Cinnamomum verum; MC: Myrtus communis; RO: Rosmarinus officinalis; SO: Salvia officinalis; SEMa: Standard Error of the Mean (Pooled).
Since only the MC Et-Uf extract was effective on S. uberis and all the replicates showed the same ZOI (9 mm, corresponding to a relative inhibitory response of 33.00%) these data were not analyzed by ANOVA. Our findings with MC are partially in agreement with those of Dulger & Gonuz [11] who observed inhibitory effects on B. cereus, S. aureus and also on P. aeruginosa by using MC ethanol, filtered (0.45 |im) extracts at 200 mg/ml and obtaining a ZOI of 20 mm for each bacterial species. In comparison with the outcomes of Dulger & Gonuz [11], a lack of effect of MC on P. aeruginosa and a smaller ZOI (about 9 mm, not shown) for B. cereus and S. aureus observed in our study were probably due to the different extraction method and lower nominal concentration (20 mg/ml) we used.
In comparison to the positive controls, with the only exception of SO, all the remaining plant extracts showed antibacterial activity against the contagious S. aureus ranging from 6.97% for MC W-F up to 37.35% for MC Et-Uf whereas the in vitro growth of S. agalactiae was inhibited only by RO Et-Uf extracts with an average relative response of 35.00%. On the other hand, the biological activity observed for RO Et-Uf extracts confirmed previous results [12,13]. Also, Ceyhan et al. [14] reported some inhibitory effects on B. cereus, S. aureus, and E. coli both with water and ethanol extracts
Figure 1: In graph were reported the effects of unfiltered (Uf), filtered (F), water (W) and ethanol (Et) MC extracts on B. cereus.Data (mean±xSE) are expressed as relative percent response respect to the positive control (Enrofloxacin). A,BP<0.001 between filtration treatments within the same extraction solvent (W or Et). ***P<0.001 between extraction solvents within filtration treatment (F or Uf)
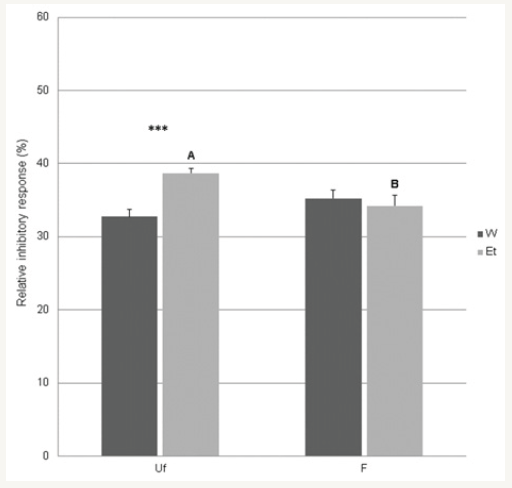
The inhibition levels of SO ethanol extract against B. cereus (Table 2) were in line with the results from Climati et al. [15] who showed biologically active filtered SO ethanol extract on the same bacterial strain. E. coli, P. aeruginosa and S. dysgalactiae were substantially unaffected by all extracts. Taken together, our and the literature findings confirm higher bio-activity of unfiltered ethanol extracts (Figure 1) and in some cases only such extracts exhibited activity to the tested bacterial strains. Al Laham & Al Fadel [13], for RO, stated that such a different efficacy could be probably related to some phenolic compounds more easily extracted by ethanol than water. With the only exception of the CV extracts, our findings confirm that most of the biologically active compounds were poorly extracted by water. With minor exceptions (i.e. CV extracts), we found that the biological activity of plant extracts was reduced by filtration, probably able to induce retention of some potentially active particles or aggregates larger than the filter size.
Conclusion
In summary, all extracts showed activity against B. cereus and S. aureus whereas SO resulted active only against B. cereus; MC and RO showed activity against S. uberis and S. agalactiae, respectively Effects of filtration on the bacterial inhibiting potential of plant extracts were evidenced. In vivo studies need to evaluate the possible use of single active extracts or a mix of active extracts in animals suffering of mastitis.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
References
- Gomes F, Henriques M (2016) Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Cur Microbiol 72(4): 377-382.
- Blowey R, Edmondson P (2010) Mastitis Control in Dairy Herds. CABI 1-272.
- Economou V, Gousia P (2015) Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 1(8): 49-61.
- Solorzano-Santos F, Miranda-Novales MG (2012) Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol 23(2): 136-141.
- Berahou A, Auhmani A, Fdil N, Benharref A, Jana M, et al. (2007) Antibacterial activity of Quercus ilex bark's extracts. Journal of ethnopharmacology 112(3): 426-429.
- Atyabi N, Vodjgani M, Gharagozloo F, Bahonar A (2006) Prevalence of bacterial mastitis in cattle from the farms around Tehran. Iran J Vet Res 7(3): 7-10.
- Oviedo-Boyso J, Valdez-Alarcon JJ, Cajero-Juarez M, Ochoa-Zarzosa A, Lopez-Meza JE, et al. (2007) Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J Infect 54(4): 399-409.
- Schmitt-van de Leemput E, Zadoks RN (2007) Genotypic and phenotypic detection of macrolide and lincosamide resistance in Streptococcus uberis. J Dairy Sci 90(11): 5089-5096.
- Bhalodia NR, Shukla VJ (2011) Antibacterial and antifungal activities from leaf extracts of Cassia fistulal. J Adv Pharm teachnology Res 2(2): 160-168.
- Joshi B, Lekhak S, Sharma A (2010) Antibacterial Property of Different Medicinal Plants: Ocimum sanctum, Cinnamomum zeylanicum, Xanthoxylum armatum and Origanum majorana. Kathmandu Univ. J Sci Eng Technol 5(1): 143-150.
- Dulger B, Gonuz A (2004) Antimicrobial Activity of Certain Plants used in Turkish Traditional Medicine. Asian J Plant Sci 3(1): 104-107.
- Keskin D, Oskay D, Oskay M (2010) Antimicrobial activity of selected plant spices marketed in the West Anatolia. Int J Agric Biol 12(6): 916920.
- Al Laham SA, Al Fadel FM (2013) Antibacterial efficacy of variety plants against the resistant streptococcus which cause clinical mastitis in cows. Asian J Pharm Res Heal Care 5(1): 32-41.
- Ceyhan N, Keskin D, Ugur A (2016) Antimicrobial activities of different extracts of eight plant species from four different family against some pathogenic microoorganisms. J Food Agric Environ 10: 193-197.
- Climati E, Mastrogiovanni F, Valeri M, Salvini L, Bonechi C, et al. (2013) Methyl carnosate, an antibacterial diterpene isolated from Salvia officinalis leaves. Nat Prod Commun 8(4): 429-430.
© 2017 Fabio Mastrogiovanni, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






