- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Avian Influenza in Egypt
Kaoud HA*
Department of Veterinary Hygiene, Cairo University, Egypt
*Corresponding author: Kaoud HA, Department of Veterinary Hygiene, Environmental Pollution and Management, Faculty of Veterinary Medicine, Cairo University, Giza 11221, Egypt
Submission: September 01, 2017; Published: November 13, 2017

ISSN : 2576-9162Volume1 Issue4
Aim
In this mini-review we have presented some ecological aspects of the isolated avian influenza viruses in Egypt since 2006, genetic characterization and the effect of some physical and chemical agents on their activity.
Abbreviations: HI: Hemagglutination Inhibition; HA: Hemagglutination; HPIIV: Highly Pathogenic Avian Influenza Virus
Introduction
HPAI is a disease of global concern because of the threat posed to food security in regions that are dependent on poultry as a main source of protein and livelihood. An additional concern is that the H5N1 virus may mutate and cause a human influenza pandemic in which millions of human lives would be threatened [1-4].
Egypt experienced the disease since the first introduction of highly pathogenic Avian Influenza HPAI H5N1 in 2006. The virus widely extended in very short time and infected commercial production sectors and backyards [5,6].
Egypt is considered a hotspot for the evolution of a pandemic potential virus either via antigenic drift of the H5N1 to increase its adaptation to humans or H9N2 or through re assortment with other IAV subtypes, especially H3N2 virus.
Material and Methods
i. Genetic characterization of Egyptian H5N1 and H9N2 viruses-Analysis of the haemagglutinin (HA) and phylogenetic tree, identified.
ii. Chicken litter from a commercial broiler farm was provided.
iii. The effect of temperature and UV light on the infectivity of avian influenza virus (Samples were divided into 4 groups as follows: The first group was tested at 28 °C. The second group was tested at 37 °C. The third group was tested at 56 °C. The last group was subjected to UV light exposure at a density of 4-5 w/cm2 (25 °C), which was the average density during the day at the study site.
iv. Treatment with disinfectants (The solution of disinfectant was added to half of the boxes of contaminated chicken litter and half of the controls).
v. Viability of the virus (The filtrate from each of the groups was inoculated into three 9-11 day-old chicken embryonated eggs according to WHO protocol. After 48hrs of incubation, the allantoic fluid was harvested and hemagglutination (HA) and hemagglutination inhibition (HI) tests were used to identify the recovered virus).
Results and Discussion
Phylogenic and genetic changes of H5 n1 and H9n2 in Egypt
Conditions that favor genetic changes in the virus via antigenic shift (mutation) and drift (re assortment) are most likely to favor the emergence of HPAI viruses. The natural tendency for influenza viruses to regularly undergo point mutations, an inherent characteristic of RNA viruses, means that new viruses are continually being produced, with differences in genetic fitness [7]. Conditions that exert selective pressure on circulating viruses at both the host and population level act to increase the rate of mutation of viruses and therefore favour the appearance and establishment of dominant virus strains [8]. Thus, intensive poultry production systems in which a continuous and easily accessible source of susceptible hosts are present are considered prime conditions under which pathogenicity may emerge. Other cited conditions have been the inadequate use of vaccinations or incomplete vaccination coverage that has allowed field strains to reassort with vaccinal strains [9].
Genetic characterization of egyptian H5N1 viruses
Analysis of the haemagglutinin (HA) phylogenetic tree identified that the viruses are fall within the clade 2.2.1.1.
Genetic characterization of egyptian H9N2 viruses
Analysis of the haemagglutinin (HA) phylogenetic tree, identified that the viruses are fall within the A, B and C groups.
Meta-analysis situation and evaluation of the epidemic studies and their measurements of AIV in Egypt
It had wide variations according to Cochran's Q statistic and Higgins and Thompson's I 2, as shown in Figures 1 & 2 with low precision will show a wide variation in effects ,while studies with high precision will show much less variation [10].
Meta-analysis situation and evaluation of the phylogenic studies of AIV in Egypt
It had low variations according to Cochran's Q statistic and Higgins and Thompson's I 2, as shown Figures 1 & 2 with high precision will show much less variation [10].
Figure 1: Effect of feeding Moringa Oleifera powder on total cholesterol level in dog’s serum.
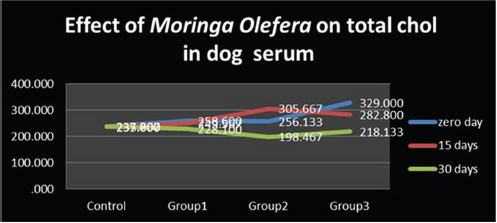
Figure 2: Effect of feeding Moringa Oleifera powder on antibody titer in dog serum vaccinated against.

Kaoud et al. [11] have presented some aspects of the highly pathogenic avian influenza virus (HPIIV) in Egypt and the effect of some physical and chemical agents on its activity. The effect of temperature and UV light on the infectivity of isolated avian influenza virus H5N1 virus in litter could be inactivated by increasing temperature above 50 °C for at least 24hr . UV light could not destroy the infectivity of the virus completely even after exposure for 48 hr.
Disinfectants were evaluated in their study including Fnvirolyte, Virkon®-S 1%, Aldekol 0.5%, and bioscentry 0.5%. The results revealed that Envirolyte was very effective in reducing the titre of H5N1 virus after 10, 30min and 12hr of exposure at 25 °C from (28) to (23) but it completely destroying the virus after 24hr of exposure at 25 °C with complete reduction in HA activity. The results also, revealed that Virkon- S® and Aldekol were effective in reducing the titre of virus after 30 min of exposure at 25 °C to (23) without any additional reduction afterwards for Virkon- S®. While, Aldekol succeeded in reducing the titre of H5N1 virus after 12hr of exposure at 25 °C to (22) but without any inactivation of HA.
Conclusion
The increase in human cases may be attributed to: increased circulation in backyard poultry, lower public health awareness of the risks, and seasonal factors such as closer proximity to birds due to cold weather. It is also suggested that co-circulation of A (H9N2) may be involved through multiple mechanisms in the enhanced spread of A(H5N1) amongst poultry in Egypt.
We think that avian influenza virus undergoes frequent antigenic drift, which is the accumulation of point mutations in the antigenic domain of the HA protein. As a result, viruses with a slightly changed antigenic structure emerge and can escape the host's acquired immunity, whether this immunity is acquired by natural infection or vaccination. Therefore, to maintain optimal protection by vaccination, the presently prevailing strains of influenza virus need to be included in each year's, influenza vaccine, requiring yearly reevaluation and frequent changes to the vaccine formulation.
For successful reduction of the negative impact on human health and associated economic and food security consequences, it will be essential to strengthen animal and human disease surveillance and disease control programmes. H5N1 virus can be inactivated in the poultry farms/ hatcheries using high temperature (e.g. 56 °C), using of disinfectants of the material to be disinfected. However, it may not be practically feasible for the farmers. Use of disinfectants seems more appropriate and practicable. Consequently there is no need to depopulate the poultry sheds after AIV outbreak for long period of time before arrival of new stock if disinfectants are used appropriately. The disinfection of surfaces also plays a role in controlling the spread of disease to other facilities. Mostly liquid disinfectants have been used for this purpose.
References
- Li KS, Guan Y, Wang J, Smith GJ, Xu KM, et al. (2004) Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430: 209-213.
- Zambon M (1999) Active and passive immunization against respiratory syncytial virus. Reviews in Medical Virology 9(4): 227-236.
- Barclay WS, Zambon M (2004) Pandemic risks from birdflu. BMJ 328: 238-239.
- WHO (World Health Organisation) (2005) WHO Global Influenza Preparedness Plan. The role of WHO and recommendations for national measures before and during pandemics. Updated November 2005. Geneva: WHO 2005. Document no. WHO/CDS/cSR/GIP/2005.5.
- Kaoud H (2007) HPAI epidemic in Egypt: evaluation, risk factors and dynamic of spreading. International Journal of Poultry Science 6: 983988.
- Aly MM, Arafa A, Hassan MK (2008) Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 52(2): 269-277.
- Horimoto T, Kawaoka Y (2001) Pandemic threat posed by avian influenza A viruses. Clin Microbiol Rev 14(1): 129-149.
- Ferguson NM, Galvani AP, Bush RM (2003) Ecological and immunological determinants of influenza evolution. Nature 422(6930): 428-433.
- Escorcia M, Vazquez L, Mendez ST, Ropon AR, Lucio E, et al. (2008) Avian influenza: genetic evolution under vaccination pressure. Virology Journal 5: 15.
- Kaoud HA, Khalf MA, Ismail TF, Ismaail EM (2016) Pro and Retrospective Epidemiological Situation of Avian Influenza in Egypt. European Journal of Academic Essays 3(7): 254-259.
- Kaoud HA, Ismail TF, Khalf MA (2016) The effect of some physical and chemical agents on the infectivity of the highly pathogenic avian influenza virus in Egypt. European Journal of Academic Essays 3(4).
© 2017 Kaoud HA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






