- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Evaluation of A Lactic Acid Based Probiotic on Leaky Gut and Microbiome Associated with Salmonella Enteritidis Infection and Feed Restriction in Broiler Chickens
D. Mahaffey1, Lucas E. Graham1, Nicole Calhoun1, Ruben Merino-Guzman2, Kyle D. Teague1, Mikayla F.A. Baxter1, Juan D. Latorre1, Amanda D. Wolfenden1, Xochitl Hernandez-Velasco2, Billy M. Hargis1, Lisa R. Bielke3, and Guillermo Tellez1*
1Department of Poultry Science, University of Arkansas, Fayetteville, USA
2Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México, Mexico
3Department of Animal Sciences, The Ohio StateUniversity, USA
*Corresponding author:Guillermo Tellez, Department of Poultry Science, Center of Excellence for Poultry Science, University of Arkansas, 1260 W Maple, POSC 0-114, Fayetteville, AR 72701, USA
Submission: September 09, 2017; Published: September 14, 2017

ISSN : 2576-9162Volume1 Issue1
Abstract
The objective of the present study was to evaluate the effect of a lactic acid based probiotic on leaky gut and microbiome associated challenges with Salmonella Enteritidis infection and 24h feed restriction (FR) in broiler chickens. Chickens were orally gavaged with 2x104cfu/chick of S. Enteritidis at 1 d of age and then were randomly assigned to one of four groups: 1) Control, 2) Probiotic control, 3) FR, and 4) Probiotic+FR. The probiotic was included in the drinking water for 16 days. Blood samples were collected for measuring leakage of FITC-d, and ceca content was also collected for microbiome evaluation. In the present study, the probiotic reduced FITC-d when compared with FR chickens without the probiotic. At Phylum level, both groups treated with probiotic had higher proportion of Firmicutes and Bacteroidetes. At the Class level, both control groups in this trial had an increase in Gammaproteobacteria. This study confirms that FR increases gut permeability in chickens, but these changes were prevented by the administration of a lactic acid based probiotic.
Keywords: Chicken; Feed restriction; Gut permeability; Microbiome; Stress
Abbreviations: FR: Feed Restriction; GIT: Gastrointestinal Tract; TJ: Tight Junction; LAB: Lactic Acid Bacteria; QIIME: Quantitative Insights into Microbial Ecology; OTUs: Operational Taxonomic Units; BWG: Body Weight Gain; GALT: Gut Associated Lymphoid Tissue; CRF: Corticotropin- Releasing Factor
Introduction
Due to intensive genetic selection, broiler chickens have become the most efficient meat-producing animals because of their fast growth, supported by a virtually unlimited voluntary feed intake. These characteristics cause many problems in the management of broiler breeder hens because of the negative correlation between muscle growth and reproduction ability. Hence, commercial restricted feeding programs in broiler breeders have been implemented, with negative effects on welfare and health, as birds are continuously hungry [1]. Previous research in poultry have showed that feed restriction (FR) increases the plasma levels of corticosterone, an accepted indicator of stress in birds, and it is associated with systemic and local inflammation in the gastrointestinal tract (GIT) as well as oxidative stress [2-4]. Oxidative stress is not only a causative factor of cellular injury but also a pivotal regulator of all crucial cellular metabolism pathways [5,6]. Directly or indirectly, oxidative stress contributes to the structural and functional derangement of the intestinal mucosa. Specifically, high levels of lipid peroxidation, protein oxidation, and glutathione redox state imbalance have been linked with disruption of gut barrier integrity through alterations of the tight junction (TJ) structural complex and enterocyte apoptosis, leading to increased intestinal permeability [7]. Similarly, recent studies conducted in our laboratory have demonstrated that the stress caused by 24h of FR [8,9] or 0.57ppm of dexamethasone in the diet of broiler chickens for six d [8] induce a significant increase in permeability of fluorescein isothiocyanate-dextran (FITC-d) in the blood circulation and is consistent with leakage from the lumen. This suggests the presence of a change in paracellular permeability rather than in transcellular transport. The purpose of the present study was to evaluate the effect of a lactic acid based probiotic on leaky gut and microbiome changes associated with S. Enteritidis infection and feed restriction in broiler chickens.
Materials and Methods
Probiotic culture
FloraMax®-B11 is a defined probiotic culture derived from gastrointestinal poultry origin that contains proprietary strains of lactic acid bacteria (LAB), selected by their in vitro ability to inhibit enteropathogens [10]. Several published studies have shown that FloraMax®-B11 increased colonization resistance to Salmonella spp. Infections [11-14], reduced idiopathic diarrhea in commercial turkey brooding houses [15], as well as increased performance and reduced costs in poultry production [11,16,17].
Bacterial strains and culture conditions
The challenge organism used in this experiment was a poultry isolate of Salmonella enterica serovar Enteritidis, bacteriophage type 13A, obtained from the USDA National Veterinary Services Laboratory, Ames, IA, resistant to 25μg/mL of novobiocin (NO, catalog no.N-1628, Sigma) and selected for resistance to 20μg/mL of nalidixic acid (NA, catalog no.N-4382, Sigma) in our laboratory. For both trials, 100μL of S. Enteritidis from a frozen aliquot was added to 10mL of tryptic soy broth (Catalog no. 22092, Sigma) and incubated at 37 °C for 8h, and passed two times every 8h to ensure that all bacteria were in log phase. Post-incubation, bacterial cells were washed three times with sterile 0.9% saline by centrifugation at 1,864×g for 10min, reconstituted in saline, quantified by densitometry with a spectrophotometer (Spectronic 20D+, Spectronic Instruments Thermo Scientific), and diluted to an approximate concentration of 10^8cfu per milliliter. Concentrations of S. Enteritidis were further verified by serial dilution and plating on brilliant green agar (Catalog no. 70134, Sigma) with NO and NA for enumeration of actual cfu used to challenge the chickens.
Animal source
In this experiment day-of-hatch male broiler chickens were obtained from Cobb-Vantress (Siloam Springs, AR, USA) and were randomly housed in heated brooder batteries, in a controlled ageappropriate environment. Birds were provided ad libitum access to water and un medicated corn-soybean diet meeting the nutritional requirements of poultry recommended by National Research Council [18]. All animal handling procedures were approved by Institutional Animal Care and Use Committee at the University of Arkansas approval number 15006 entitled “Development of enteric inflammation models for investigation of antibiotic alternatives in poultry”.
Serum determination of FITC-d leakage
Blood samples were collected from the femoral vein, kept at room temperature for 3h, and centrifuged (500xg for 15min) to separate the serum from the red blood cells. FITC-d levels of diluted serum samples (1:5 PBS) were measured at excitation wavelength of 485 nm and an emission wavelength of 528nm with a Synergy HT, Multi-mode microplate fluorescence reader (BioTek Instruments, Inc., Vermont, USA). Fluorescence measured was then compared to a standard curve with known FITC-d concentrations. Gut leakage for each bird was reported as ng of FITC-d per mL of serum.
Experimental design
Day-of-hatch chickens were randomly assigned to one of four groups (n=20/group), neck tagged, individually weighed and placed into battery cages. Experiment groups included: 1) Control no FR; 2) Probiotic in drinking water for 16 d no FR; 3) 24 h of FR; 4) Probiotic in drinking water for 16 d plus 24 h of FR. All chickens were orally gavaged with 2x104 cfu/chick of S. Enteritidis at 1 d of age. Chickens were placed into battery cages in a controlled age-appropriate environment with unrestricted access to feed and water. Beginning at 15 d, chickens in the no FR groups, were allowed to continue with ad libitum access to feed, while chickens in FR groups, were subjected to 24h of feed withdrawal. At 16 d of age, chickens in all groups were weighed and given one dose of FITC-d (4.16mg/kg of body weight) by oral gavage. After 2.5h, they were humanely killed by CO2 asphyxiation. Blood samples were collected for measuring leakage of FITC-d, and in this trial, cecal contents were also taken for DNA extraction as described below.
Ceca Microbial population assessment
Ceca contents (200mg) from each bird were collected for DNA isolation utilizing QIA amp DNA Stool Mini Kit (Qiagen, Valencia, CA). The concentration of extracted DNA was diluted to 10ngμL-1 for the preparation of a sequencing library targeting the V4 region of 16S rRNA gene. Isolated DNA samples were amplified via a PCR using dual-index primers and normalized the amplicons with a Sequal PrepTM Normalization kit (Life Technology, Carlsbad, CA) according to the manufacturers’ recommendation. The library was constructed by combining 5μL of each normalized aliquot samples for further assessment. Library concentration and product size were confirmed using a KAPA Library Quantification Kit (Kapa Biosystems, Woburn, MA) via a quantitative PCR (qPCR, Eppendorf, Westbury, NY) and an Agilent 2100 Bioanalyzer system (Agilent, Santa Clara, CA), respectively. The 20nM of pooled library aliquot and the 20nM of PhiX control v3 were combined with 0.2N fresh NaOH and HT1 buffer and mixed a second time with 5% of the PhiX control v3. The 600μL of the mixture containing pooled library, PhiX control v3, NaOH and HT1 buffer was subsequently loaded onto a MiSeq v2 reagent cartridge to run sequencing.
Microbiome sequencing analysis by qiime pipeline
Raw sequencing read files were processed using quantitative insights into microbial ecology (QIIME) pipeline (version 1.9.0). Each of the operational taxonomic units (OTUs) was assigned to specific microorganisms to determine taxonomic levels and subjected to alpha and beta diversity analyses and tables were constructed by clustering sequences with 97% or higher identity based on Greengenes 16S rRNA gene database. In addition, OTUs that were not observed at least twice were excluded manually to eliminate possible erroneous reads from sequencing. Chimeras that were sequences generated by multiple templates or parent sequences were identified and filtered by ChimeraS layer script that utilizes BLAST. Also, the OTU table was sub sampled or rarefied using a minimal observed OTU value to discard any samples that have unusually fewer sequences. Subsequently, OTUs tables were converted to taxonomic tables for further analysis. Weighted and unweighted version of UniFrac graphs and rarefaction plots were generated for beta and alpha diversity test respectively.
Data and statistical analysis
Body weight gain (BWG), serum FITC-d concentration and proportion of bacterial composition were subjected to analysis of variance as a completely randomized design, using the General Linear Models procedure of SAS [19]. Significant differences among the means were determined by Duncan’s multiple-range test at P<0.05.
Results
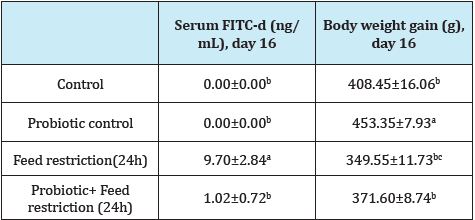
Table 1 shows the results of the effect of a lactic acid bacteria probiotic on serum FITC-d associated with 24h feed restriction and S. Enteritidis infection in broiler chickens. In the present study, control and probiotic chickens with no FR showed no leakage of FITC-d. However, a significant reduction in serum FITC-d concentration was observed in chickens that received the probiotic and were exposed to 24h of FR at d 16 when compared with FR control non-treated chickens. Interestingly, a significant increase in BWG was observed in chickens that received the probiotic when compared with control chickens without the probiotic. Chickens that received the probiotic and FR for 24 h showed a numerical increase in BWG when compared with FR control chickens (Table 1).
Table 1: Effect of a lactic acid bacteria probiotic on serum FITC-d associated with Salmonella enteritidis infection and 24 hours feed restriction in broiler chickens.
On day 1, chickens were orally gavaged with 2 x104 Salmonella Enteritidis
Data expressed as mean±standard error.
a-cMeans within a column with different superscripts differ (P<0.05), n=20 chickens/group
The results of the effect of a lactic acid bacteria probiotic on Phylum distribution (cumulative % lowest common ancestor) and Class direct assignment in % for all ceca samples of broiler chickens associated with S. Enteritidis infection and 24h feed restriction are summarized in Table 2. At the Phylum level microbiome analysis, both groups of chickens treated with the probiotic had the higher proportion of Firmicutes, followed by control chickens with no FR and chickens that received FR had the lowest number of Firmicutes. Control chickens with no FR and chickens that received FR also showed the higher numbers of Bacteroidetes, followed by both groups that received the probiotic. Control chickens with no FR had the lowest numbers of Bacteroidetes but also had the higer proportion of Proteobacteria. Proportion of Actinobacteria was very low in all groups with no significant differences among them. At the class level, it was interesting to observe that both the control and 24h feed restriction group in this trial had an increase in Gammaproteobacteria, when compared with both groups that received the probiotic, but Clostridia and Betaproteobacteria proportions were similar in all groups (Table 2).
Table 2: EEffect of a lactic acid bacteria probiotic on Phylum distribution (cumulative percentage lowest common ancestor) and class direct assignment in percentage for all ceca samples of broiler chickens associated with Salmonella Enteritidis infection and 24 hours feed restriction.

a-bSuperscripts within rows indicate significant difference at P<0.05, n = 6.
Discussion
Chronic FR represents a permanent stress for any organism, particularly for poultry with relatively high metabolic requirements, where increased plasma corticosterone concentrations are often associated with chronic stress observed in FR programs [20,21]. Stress can induce a variety of changes in normal gastrointestinal function, including changes in gut motility and permeability, as well as alterations in ion, fluid, and mucus secretion and absorption [22- 25]. Animal models of acute and chronic stress demonstrate that stress induces changes in intestinal barrier function increasing transcellular and paracellular intestinal permeability associated with a temporary redistribution of TJ proteins [26-30]. These changes have been linked to Mast cells who are important effectors of the brain-gut axis that translate the stress signals into the release of a wide range of neurotransmitters and proinflammatory cytokines, with dramatic effects on gastrointestinal physiology [31,32]. Since the mucosal barrier of the GIT represents the largest body surface in contact with the external environment, this fragile barrier plays a crucial role in the biology of metazoans [6,33,34]. The single line of intestinal epithelial cells (IECs), are responsible of maintaining its selective barrier function through the formation of complex protein networks: desmosomes, adherent junctions, and TJ [35-37]. Tight juntions are molecules that prevent paracellular permeability [38,39]. Intestinal epithelial cells play important roles in mechanisms of innate immunity as part of the gut associated lymphoid tissue (GALT), displaying a wide array of immune functions such as pathogen recognition, release of anti-microbial compounds, and secretion of several hormones, neurotransmitters, enzymes, cytokines and chemokines [40-42]. Furthermore, IECs such as goblet cells secrete several mucins that reinforce the overall intestinal barrier [43,44]. Therefore, any injury to IECs could lead to dramatic changes in gut permeability that result in disruption of the GIT homeostasis, followed by intestinal and systemic inflammation [45]. Published studies have shown the mechanisms linked with the disruption of TJ by inflammatory mediators, among them: hormones, oxygen free radical species, enzymes as well as multiple proinflammatory cytokines released by pathogens, diet ingredients, or stress [39,46].
More recently, we have demonstrated that a rye-soybean ration, 24 h of feed restriction, or dietary administration of dexamethasone induce leaky gut, bacterial translocation, and dysbacteriosis in broiler chickens [8,47,48]. In the present study, the commercial probiotic was able to reduce the intestinal permeability of FITC-d into the serum in models previously published of FR (Table 1). FITC-d is a large molecule (3-5kDa) which does not usually leak through the intact gastrointestinal tract barrier. Nevertheless, when epithelial TJ are disrupted, serum FITC-d leaks to the blood stream as demonstrated by an increase in trans-mucosal permeability associated with the stress caused by 24 h of FR [49]. As previously reported, the fact that gut permeability was significantly higher in FR chickens suggests that this stress practice has a strong impact on the epithelial barrier, altering gut permeability in broiler chickens [8,50]. On the other hand, it has been studied in chickens that elevated serum concentrations of corticosteroid are associated with environmental stress [51-54]. The stress-induced intestinal disturbances caused by corticosteroids is mediated by corticotropin-releasing factor (CRF), which increases intestinal paracellular permeability via mast cell dependent release of TNF-α and proteases causing systemic and local inflammation in the GIT by oxidative stress [1-3,37,55-58]. Oxidative stress is not only a causative factor of cellular injury but also a pivotal regulator of all crucial cellular metabolic processes [5,6]. Directly or indirectly, oxidative stress contributes to the structural and functional derangement of the intestinal mucosa. Specifically, high levels of lipid peroxidation, protein oxidation, and glutathione redox state imbalance have been linked with disruption of gut barrier integrity through alterations of the TJ structural complex and enterocyte apoptosis leading to increased intestinal permeability [7,59,60]. In the present study, in addition to the stress caused by FR, all chickens were also challenged with S. Enteritidis at day-of-hatch. Similar to a previous study conducted by our laboratory using S. Heidelberg [61], in the present study S. Enteritidis challenge did not increase the leakage of FITC-d (Table 1) by itself. Metagenomic analysis of cecal content using the MEGAN software can be used to interactively analyze and compare metagenomic and metatranscriptomic data, thereby providing a percent identity filter that can be used to enforce the following levels of percentage sequence identities for an assignment at a given taxonomic level [62].
Chickens treated with the probiotic had the higher proportion of Firmicutes, followed by control chickens with no FR and chickens that received FR had the lowest numbers. Control chickens with no feed restriction and chickens that only received FR also showed the higher numbers of Bacteroidetes, followed by both groups that recived the probiotic. Control chickens with no FR had the lowest numbers of Bacteroidetes, however, these chickens also had the higher proportion of Proteobacteria. Control chickens with no FR had the lowest numbers of Bacteroidetes but had the higher proportion of Proteobacteria. Proportion of Actinobacteria was very low in all groups with no significant differences among them. At the Class level, it was interesting to observe that both control groups in this trial had an increase in Gammaproteobacteria, when compared with both groups that received the probiotic, but Clostridia and Bacilli proportions were similar in all groups. Changes in the proportion of Phylum and Class were associated with the challenge of S. Enteritidis which belongs to phylum Proteobacteria, class Gamma proteobacteria (Table 2). In contrast, chickens that received the probiotic had the highest proportion of Firmicutes and Bacteroidetes, but the lowest amount of Proteobacteria. Probiotic treated chickens also showed significant reduction in Gamma proteobacteria, but similar to the control group, a higher proportion in Clostridia and Bacilli. The shift in these bacterial populations, had a similar trend as previously reported with S. Heidelberg in previous research [63].
The results of the present study confirm that 24h of FR to broiler chickens increased gut permeability as was indicated by the detection of FITC-d in the serum, but these changes were prevented by the administration of a lactic acid based probiotic. In addition to their well-recognized immune modulator properties [64-68] several investigators have reported that both lactic acid based probiotics as well as Bacillus sp. based probiotics maintain intestinal homoeostasis and improve the integrity of the intestinal epithelial cells through their anti-inflammatory and anti-oxidative properties [69-71]. Studies to evaluate anti-inflammatory and antioxidant properties of previously selected probiotic in chickens under different stress conditions are currently being evaluated [72- 76].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgement
Arkansas Bioscience Institute under the project: Development of an avian model for evaluation early enteric microbial colonization on the gastrointestinal tract and immune function.
References
- Decuypere E, Bruggeman V, Everaert N, Li Y, Boonen R, et al. (2010) The broiler breeder paradox: ethical, genetic and physiological perspectives, and suggestions for solutions. Br Poult Sci 51(5): 569-579.
- De Jong IC, van Voorst AS, Blokhuis HJ (2003) Parameters for quantification of hunger in broiler breeders. Physiol Behav 78(4-5): 773-783.
- Khajavi M, Rahimi S, Hassan ZM, Kamali MA, Mousavi T (2003) Effect of feed restriction early in life on humoral and cellular immunity of two commercial broiler strains under heat stress conditions. Br Poult Sci 44(3): 490-497.
- Abu-Dieyeh ZHM (2006) Effect of chronic heat stress and long-term feed restriction on broiler performance. Int J Poult Sci 5(2): 185-190.
- Lied GA, Milde AM, Nylund K, Mujic M, Grimstad T, et al. (2012) Increased wall thickness using ultrasonography is associated with inflammation in an animal model of experimental colitis. Clin Exp Gastroenterol 5: 195- 201.
- Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH (2013) Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int 33(10): 1457-1469.
- Karavolos MH, Spencer H, Bulmer DM, Thompson A, Winzer K, et al. (2008) Adrenaline modulates the global transcriptional profile of Salmonella revealing a role in the antimicrobial peptide and oxidative stress resistance responses. BMC Genomics 9: 458.
- Kuttappan VA, Vicuña EA, Latorre JD, Wolfenden AD, Téllez GI, et al. (2015) Evaluation of gastrointestinal leakage in multiple enteric inflammation models in chickens. Front Vet Sci 2: 66.
- Kuttappan VA, Berghman LR, Vicuña EA, Latorre JD, Menconi A, et al. (2015) Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult Sci 94(6): 1220-1226..
- Menconi A, Kallapura G, Latorre JD, Morgan MJ, Pumford NR, et al. (2014) Identification and characterization of lactic acid bacteria in a commercial probiotic culture. Biosci Microbiota Food Health 33(1): 25-30.
- Torres-Rodriguez A, Higgins SE, Vicente JL, Wolfenden AD, Gaona- Ramirez G, et al. (2007) Effect of lactose as a prebiotic on turkey body weight under commercial conditions. J Appl Poult Res 16(4): 635-641..
- Vicente J, Wolfenden A, Torres-Rodriguez A, Higgins S, Tellez G (2007) Effect of a Lactobacillus species-based probiotic and dietary lactose prebiotic on turkey poult performance with or without Salmonella enteritidis challenge. J Appl Poult Res 16(33): 361-364.
- Tellez G, Pixley C, Wolfenden RE, Layton SL, Hargis BM (2012) Probiotics/ direct fed microbials for Salmonella control in poultry. Food Res Int 45(2): 628-633.
- Menconi A, Reginatto AR, Londero A, Pumford NR, Morgan M, et al. (2013) Effect of organic acids on Salmonella Typhimurium infection in broiler chickens. Int J Poult Sci 12: 72-75.
- Higgins SE, Torres-Rodriguez A, Vicente JL, Sartor CD, Pixley CM, (2005) Evaluation of intervention strategies for idiopathic diarrhea in commercial turkey brooding houses. J Appl Poult Res 14(2): 345-348.
- Vicente JL, Torres-Rodriguez A, Higgins SE, Pixley C, Tellez G, et al. (2008) Effect of a selected Lactobacillus spp.-based probiotic on Salmonella enterica serovar Enteritidis-infected broiler chicks. Avian Dis 52(1): 143-146.
- Biloni A, Quintana CF, Menconi A, Kallapura G, Latorre J, et al. (2013) Evaluation of effects of EarlyBird associated with FloraMax-B11 on Salmonella Enteritidis, intestinal morphology, and performance of broiler chickens. Poult Sci 92(9): 2337-2346.
- National Research Council (1994) Nutrient Requirements of Poultry. (9th edn), National Academic Press, Washington, USA.
- SAS Institute Inc (2002) SAS User Guide. Version 9.1. Cary, NC: SAS Institute Inc.
- Washburn KW, Peavey R, Renwick GM (1980) Relationship of strain variation and feed restriction to variation in blood pressure and response to heat stress. Poult Sci 59(11): 2586-2588.
- Messias de Bragan ca M, Mounier AM, Prunier A (1998) Does feed restriction mimic the effects of increased ambient temperature in lactating sows? J Anim Sci 76(8): 2017-2024.
- Alverdy J, Aoys E (1991) The effect of glucocorticoid administration on bacterial translocation. Evidence for an acquired mucosal immunodeficient state. Ann Surg 214(6): 719-723.
- Collins SM, Bercik P (2009) The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterol 136(6): 2003-2014.
- Verbrugghe E, Boyen F, Van Parys A, Van Deun K, Croubels S, et al. (2011) Stress induced Salmonella Typhimurium recrudescence in pigs coincides with cortisol induced increased intracellular proliferation in macrophages. Vet Res 42: 118.
- Karavolos MH, Winzer K, Williams P, Khan CM (2013) Pathogen espionage: multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol Microbiol 87(3): 455-465.
- Maejima K, Deitch E, Berg RD (1984) Bacterial translocation from the gastrointestinal tracts of rats receiving thermal injury. Infect Immun 43(1): 6-10.
- Koh TS, Peng RK, Klasing KC (1996) Dietary copper level affects copper metabolism during lipopolysaccharide-induced immunological stress in chicks. Poult Sci 75(7): 867-872.
- Matter K, Balda MS (2007) Epithelial tight junctions, gene expression and nucleo-junctional interplay. J Cell Sci 120(9): 1505-1511.
- Assimakopoulos SF, Gogos C, Labropoulou-Karatza C (2011) Could antioxidants be the “magic pill” for cirrhosis-related complications? A pathophysiological appraisal. Med Hypotheses 77(3): 419-423.
- Ilan Y (2012) Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol 18(21): 2609-2618.
- Groschwitz KR, Hogan SP (2009) Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clinic Immunol 124(1): 3-20.
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, et al. (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25(3): 397-407.
- Seki E, Schnabl B (2012) Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 590(3): 447-458.
- Galley JD, Bailey MT (2014) Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes 5(3): 390-396.
- Schneeberger EE, Lynch RD (1992) Structure, function, and regulation of cellular tight junctions. Am J Physiol 262(6 pt 1): L647-L661.
- Assimakopoulos SF, Papageorgiou I, Charonis A (2011) Enterocytes’ tight junctions: from molecules to diseases. World J Gastrointest Pathophysiol 2(6): 123-137.
- Stumpf MT, Fischer V, McManus CM, Kolling GJ, Zanela MB, et al. (2013) Severe feed restriction increases permeability of mammary gland cell tight junctions and reduces ethanol stability of milk. Animal 7(7): 1137- 1142.
- Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, et al. (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298(6): G851–G859.
- Hu YJ, Wang YD, Tan FQ, Yang WX (2013) Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep 40(11): 6123-6142.
- Ballard ST, Hunter JH, Taylor AE (1995) Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr 15: 35-55.
- Alverdy J, Zaborina O, Wu L (2005) The impact of stress and nutrition on bacterial-host interactions at the intestinal epithelial surface. Curr Opin Clin Nutr Metab Care 8(2): 205-209.
- Edelblum KL, Turner JR (2009) The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol 9(6): 715- 720.
- Sakamoto K, Hirose H, Onizuka A, Hayashi M, Futamura N, et al. (2000) Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J Surg Res 94(2): 99-106.
- Johansson ME, Gustafsson JK, Sjӧberg KE, Petersson J, Holm L, et al. (2010) Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PloS One 5(8): e12238.
- Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, et al. (2009) Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci 1165: 294-300.
- Steed E, Balda MS, Matter K (2010) Dynamics and functions of tight junctions. Trends Cell biol 20(3): 142-149.
- Tellez G, Latorre JD, Kuttappan VA, Hargis BM, Hernandez-Velasco X (2015) Rye affects bacterial translocation, intestinal viscosity, microbiota composition and bone mineralization in turkey poults. PloS One 10(4): e0122390.
- Latorre JD, Hernandez-Velasco X, Bielke LR, Vicente JL, Wolfenden R, et al (2015) Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet. Br Poult Sci 56(6): 723-732.
- Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, et al. (2009) Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PloS One 4(6): e6073.
- Vicuña EA, Kuttappan VA, Tellez G, Hernandez-Velasco X, Seeber-Galarza R, et al. (2015) Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult Sci 94(6): 1353-1359.
- Gross WB, Siegel HS (1983) Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis 27(4): 972-979.
- Huff GR, Huff WE, Balog JM, Rath NC (1999) Sex differences in the resistance of turkeys to Escherichia coli challenge after immunosuppression with dexamethasone. Poult Sci 78(1): 38-44.
- Shini S, Shini A, Huff GR (2009) Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiol Behav 98(1-2): 73-77.
- Wideman RFJr, Pevzner I (2012) Dexamethasone triggers lameness associated with necrosis of the proximal tibial head and proximal femoral head in broilers. Poult Sci 91(10): 2464-2474.
- Zulkifli I, Che Norma M, Israf DA, Omar AR (2000) The effect of early age feed restriction on subsequent response to high environmental temperatures in female broiler chickens. Poult Sci 79(10): 1401-1407.
- Taché Y, Perdue MH (2004) Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil 16(1): 137-142.
- Teitelbaum AA, Gareau MG, Jury J, Yang PC, Perdue MH (2008) Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am J Physiol Gastrointest Liver Physiol 295(3): G452–G459.
- Overman EL, Rivier JE, Moeser AJ (2012) CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PloS One 7(6): e39935.
- Hangalapura BN, Nieuwland MGB, De Vries Reilingh G, Buyse J, Van Den Brand H, (2005) Severe feed restriction enhances innate immunity but suppresses cellular immunity in chicken lines divergently selected for antibody responses. Poult Sci 84(10): 1520-1529.
- Farnell MB, Donoghue AM, De Los Santos FS, Blore PJ, Hargis BM, et al. (2006) Upregulation of oxidative burst and degranulation in chicken heterophils stimulated with probiotic bacteria. Poult Sci 85(11): 1900- 1906.
- Morales-Barrera E, Calhoun N, Lobato-Tapia JL, Lucca V, Prado-Rebolledo O, et al. (2016) Risks involved in the use of enrofloxacin for Salmonella enteritidis or Salmonella Heidelberg in commercial poultry. Front Vet Sci 3: 72.
- Huson DH, Weber N (2013) Microbial community analysis using MEGAN. Methods Enzymol 531: 465-485.
- Menconi A, Wolfenden AD, Shivaramaiah S, Terraes JC, Urbano T, et al. (2011) Effect of lactic acid bacteria probiotic culture for the treatment of Salmonella enterica serovar Heidelberg in neonatal broiler chickens and turkey poults. Poult Sci 90(3): 561-565.
- Wilks M (2007) Bacteria and early human development. Early Hum Dev 83: 165-170.
- Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44(1): 26-46.
- Dimitrov DV (2011) The human gutome: nutrigenomics of the hostmicrobiome interactions. OMICS 15(7-8): 419-430.
- DLyte M (2011) Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 33(8): 574-581.
- DHowarth GS, Wang H (2013) Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients 5(1): 58-81.
- Sherman PM, Ossa JC, Johnson-Henry K (2009) Unraveling mechanisms of action of probiotics. Nutr Clin Pract 24(1): 10-14.
- Zhou Y, Qin H, Zhang M, Shen T, Chen H, et al. (2010) Lactobacillus plantarum inhibits intestinal epithelial barrier dysfunction induced by unconjugated bilirubin. Br J Nutr 104(3): 390-401.
- Zhou YK, Qin HL, Zhang M, Shen TY, Chen HQ, et al. (2012) Effects of Lactobacillus plantarum on gut barrier function in experimental obstructive jaundice. W J Gastroenterol 18(30): 3977-3991.
- Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: 141-145.
- Degnan PH, Ochman H (2012) Illumina-based analysis of microbial community diversity. ISME J 6: 183-194.
- Higgins JP, Higgins SE, Vicente JL, Wolfenden AD, Tellez G, (2007) Temporal effects of lactic acid bacteria probiotic culture on Salmonella in neonatal broilers. Poult Sci 86(8): 1662-1666.
- Tellez G, Latorre JD, Kuttappan VA, Kogut MH, Wolfenden A, et al. (2014) Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front Genet 5: 339.
- Vicuña EA, Kuttappan VA, Galarza-Seeber R, Latorre JD, Faulkner OB, et al. (2015) Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poult Sci 94(9): 2075-2080.
© 2017 D. Mahaffey, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






