- Submissions

Full Text
Aspects in Mining & Mineral Science
Recovery of Sulfur, Copper, and Gold by Reprocessing Old Flotation Tailings at Bor, Serbia
Erdem Özdemir*, Aleksandra Lang, Juha Saari and Jussi Liipo
Metso Outotec Research Center, Finland
*Corresponding author:Erdem Özdemir, Metso Outotec Research Center, Kuparitie 10, FI- 28101, Pori, Finland
Submission: December 18, 2023: Published: January 11, 2024

ISSN 2578-0255Volume12 Issue2
Abstract
Mining and processing tailings often contain significant amounts of valuable metals, that can represent valuable sources of secondary raw materials. Especially this is case in early-stage operations, in which the head grades were higher, and the tailings were higher grade. These tailings can also present a substantial risk to the environment. Serbia has copper deposits which have been exploited since ancient times, and these operations have generated large amounts of mineral processing tailings. The main objective of this study is to show how valuables can be recovered from chemically and mineralogically challenging tailings. After detailed chemical and mineralogical characterization, the laboratory scale flotation tests focused on evaluating the effect of particle size, different types of collectors, pH, and pulp potential. Based on the test work, copper and gold can be recovered effectively into pyrite concentrate.
Keywords:Copper; Gold; Mineralogy; Tailings; Flotation; Value recovery; Modeling
Abbreviations: EIT: European Institute of Innovation and Technology; RIS-CuRE: Regional Innovation Scheme-Zero Waste Recovery of Copper Tailings in the ESEE region; ICP-OES: Inductively Coupled Plasma Optical Emission Spectrometry; EMC: Element-to-Mineral Conversion; XRD: X-Ray Diffractometer; SHE: Standard Hydrogen Electrode
Introduction
Efficient reprocessing of tailings, i.e., mine waste, is crucial to the elimination of environmental risks connected with tailing ponds, such as tailings dam failure and the generation of acid mine drainage. In the meantime, the high quantity of mineral processing tailings provides an economical source of metals that can be considered secondary deposits [1]. Tailings also represent a valuable source of secondary raw materials. Therefore, tailings are nowadays considered globally as potentially exploitable and cost-saving raw material sources. This potential is because they consist of already mined and comminuted minerals [2-6].
The current research is part of the European Institute of Innovation and Technology (EIT) funded RIS-CuRE (Regional Innovation Scheme-Zero waste recovery of copper tailings in the ESEE region) project, which aims at extraction of valuable metals from old tailings and utilization of cleaned tailings. Serbia has copper deposits that have been exploited since ancient times, especially in Bor (a district in the eastern part of Serbia), dating back to 4500 BC. The Bor copper deposits mainly consist of 600 metric tons of porphyry and 200 metric tons of high sulfidation zone [7,8]. These operations have generated large amounts of mineral processing tailings. The Bor copper mine started exploiting the high-grade (17% Cu) copper ores in 1903 [9]. Open-pit mining began in 1912 and continued until 1986 with decreasing copper grades [10]. Approximately 700 million metric tons of waste rock and tailings were disposed of in the Bor Valley [11]. The dumped tailings are highly acidic, with pH values ranging between 2 and 4.25 [12], due to the decomposition of an abundance of pyrite, which has led to acid generation [13,14].
Overall, a century of mining has left its mark on the landscape of the Bor area, one of Serbia’s most polluted places [10]. The soil surrounding the old flotation tailings pond is contaminated with metallic elements containing exceptionally high concentrations of iron, copper, and arsenic [15,16]. The acidic conditions facilitate the mobility and bioavailability of toxic heavy metals. Therefore, the outcomes of this study are important to save the Bor Valley from the ongoing pollution. In the case of a tailings dam failure, there is the potential for a significant portion of the toxic material to run directly into the Borska River and onward into the Danube River, resulting in enormous environmental consequences for the entire region [12,13].
Reprocessing the tailings can reduce acid generation and the subsequent release of metals to waterways and soils by removing pyrite and simultaneous recovery of valuable metals [17]. Several studies have been conducted on laboratory scale to recover copper and gold from the Bor tailings by acid leaching [11,18]. Acid leaching followed by flotation of leaching residue [19] led to copper and sulfur recoveries of 70% and 77%, respectively. Conić et al. [18] concluded that the most efficient technology for copper recovery from the old flotation tailings of the Bor copper mine was leaching with biogenic lixiviant. Falagán et al. [16] achieved copper recoveries of over 90% by bioleaching. Bulatovic [20] found that bulk flotation is typically used in cases where the ore is acidic and the pyrite content does not exceed 15% by weight. Markovic et al. [12] and Han et al. [21] used bulk sulfide flotation to recover valuable metals from the Bor tailings. Markovic et al. [12] achieved a copper recovery of over 97% and a pyrite recovery of over 87% with bulk flotation in medium alkaline pulp conditions. The copper and sulfur grades in the bulk concentrate were 1.34% and 42.74%, respectively. Moreover, recoveries of gold and silver were above 60%. Similarly, Leppinen et al. [22] used bulk sulfide flotation to remove sulfur and recover valuable pyrite-rich tailings to control acid mine drainage. Quartz and clay minerals are common gangues of sulfide-type ore bodies and their tailings [23]. Rao et al. [24] found that sodium silicate is one of the most valuable depressants for silica gangues and that it can increase the selectivity of pyrite flotation.
Materials and Methods
Materials
The old Bor flotation tailings were disposed of in the Bor River valley from 1933 to 1987 in two tailings ponds covering an area of approximately 1.6km2. The ponds contain 27 million metric tons of tailings with an average range of 0.2-0.3% copper and 0.3-0.6ppm of gold [25]. As part of the RIS-CuRE project [26], a sample of approximately 230kg of tailings was taken from the pond by drilling and delivered for characterization and beneficiation experiments to Metso Research Center, Pori, Finland. The sample was homogenized by shoveling and divided into subsamples by a rotating sample divider.
Methods
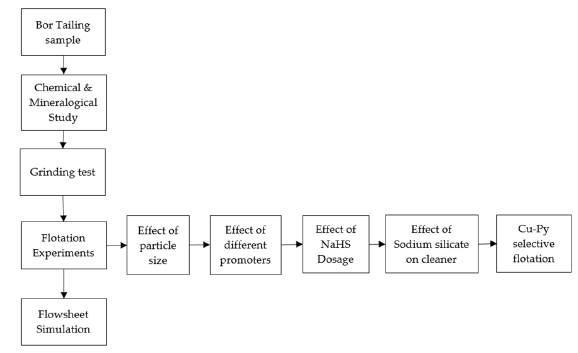
The methods used consisted of four main steps, including a chemical and mineralogical study, grinding test, flotation experiments, and flowsheet simulation, as shown in Figure 1.
Figure 1:Overall diagram of the methodology.
Chemical composition of tailings
After total dissolution, the chemical analysis of metals in solids was conducted using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, iCAP 6000, Thermo Scientific, Netherlands) . The total carbon and sulfur concentrations were deter-mined by the combustion method (CS-2000, Eltra, Germany). The gold content was determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Thermo Scientific , Netherlands).
A four-step selective dissolution method was performed on the sized and bulk samples to quantify different copper minerals, according to the procedure described by Young [27] and further developed by Metso. The selective dissolution method was carried out by analyzing the filtrated sample in dissolution by ICP-OES, with the residual continuing onto the next dissolution. The steps were as follows: P1) H2O dissolution, P2) H2SO4 dissolution, P3) KCN dissolution, and P4) HNO3SO4>+Br2 dissolution. Based on this method, copper sulfates, copper oxides and carbonates, secondary copper sulfides, and primary chalcopyrite can be distinguished, and their amounts calculated using the Element-to-Mineral Conversion (EMC) method.
Mineral composition of tailings
The mineralogical sample was screened using sieves from 600μm to 20μm in size. The P80 was calculated as 116μm and the P50 as 29μm. Seven polished resin mounts were prepared from the size fraction samples and from one bulk sample for the mineralogical studies. Due to the small amount of material in the fractions of -600μm to +212μm they were combined into one size fraction sample. The polished samples were first studied in reflected light using a Axio Imager M2m, (Zeiss, Germany) optical microscope and were later coated with a layer of approximately 10nm carbon for electron microscopy studies.
The electron microscopy measurements were performed using a field emission scanning electron microscope (FEG-SEM, JSM 7000F, JEOL Japan) equipped with an Oxford Instruments energydispersive X-ray spectrometer (EDS, X-Max 80, United Kingdom). The imaging and the EDS analyses were performed under routine conditions using 20kV acceleration voltage and a beam current of 1nA. The mineral amount was calculated based on the EDS analyses of the minerals and the analyzed chemical composition of the size fractions using the Element-to-Mineral (EMC) Calculation method [28,29]; this is included in the HSC Chemistry® 10 software [30]. Additionally, the main minerals were identified from two powder samples: original unwashed and -20μm size fraction samples, using a Aeris X-Ray Diffractometer (XRD, Malvern Panalytical, United Kingdom) with Co Kα radiation at 40kV and 15mA, using step size of 0.0110 °2θ and a step time of 48.1950s.
Mineral liberation analysis
Liberation measurements were performed using FEG-SEMEDS coupled with the Aztec Mineral liberation analysis software developed by Oxford Instruments. Mineral liberation measurements were carried out, focusing on sulfide minerals.
Grinding tests
Wet grinding tests were performed using a mild steel laboratory ball mill manufactured state technical research institute in Finland (215mm heightx205mm diameter) using 27mm (3.3kg) and 19mm (8.7kg) balls with 65% w/w (weight by weight) solids ratio. Grinding times of 5, 10, 20, 40, 80, and 105 minutes were selected to create ore grindability data for the flotation experiments.
Flotation experiments
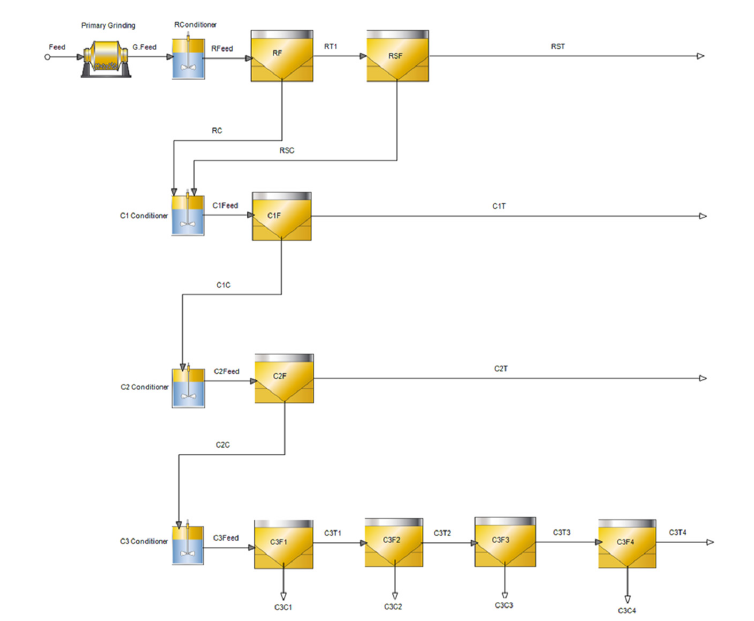
The Bor flotation experiments were carried out using a laboratory-type Outotec GTK Lab Cell (Finland) flotation machine at Metso Research Center, Pori, Finland. The GTK flotation lab cell is equipped with an adjustable external water pump to maintain a constant pulp level. Froth is recovered using an automatic froth scraper system. The flotation and grindability experiments were conducted with locally sourced tap water. The pH of the slurry was measured as 3.5. The Bor bulk flotation experiments were carried out at a 35% w/w solids ratio using 1kg and 2kg feed in 2- and 4-liter flotation cells, respectively. Moreover, 2kg feeds were used for the cleaner flotation experiments to maintain enough material. Makeup water was added during the flotation experiments to provide a constant pulp level in the flotation cell. The airflow was set to a steady 3L/min throughout the flotation experiment. The air was fed in with a 15-20 second delay to build froth. Once the froth reached the discharge lip, the flotation time and froth scraping were recorded. Impeller rotational speeds of 1300 and 1500rpm were used in the 2- and 4-liter flotation cells. Rougher flotation concentrates were collected at 2, 6, 14, 22, and 30 minutes to determine the flotation kinetics. The error of the flotation experiments was determined as 2.3% especially for the bulk stage. Flowsheets for the rougher, cleaner, and the selective flotation experiments are shown in Figures 2-4, respectively.
Figure 2:Flowsheet for kinetic rougher flotation RC1-5 refers to rougher concentrates 1 to 5. RF-Rougher Flotation, RC- Rougher Concentrate, RT- Rougher Tailings.
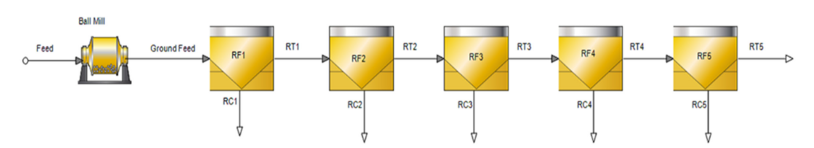
Figure 3:Flowsheet for kinetic bulk cleaner flotation experiments. RF-Rougher Flotation. RSF-Rougher Scavenger Flotation, RT-Rougher Tailings, RC-Rougher Concentrate, RSC-Rougher Scavenger Concentrate, BUC1-Bulk First Cleaner Flotation, BUC1C-Bulk First Cleaner Concentrate, BUC1T-Bulk First Cleaner Tailings.
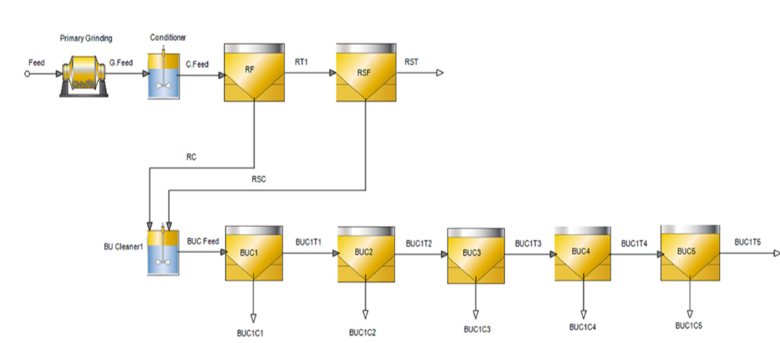
Figure 4:Flowsheet for copper pyrite selective flotation experiments
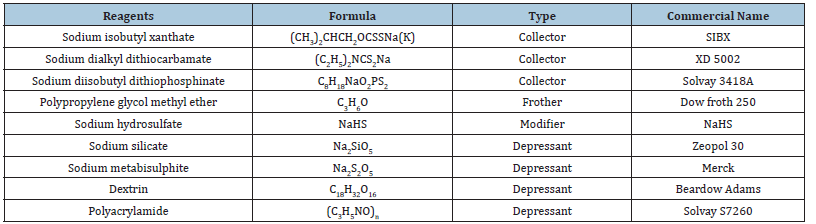
Sulfidation was applied in the bulk flotation experiments by adding sodium hydrosulfide (NaHS) stagewise. Additionally, the pulp potential (Eh) was monitored with a Radiometer brand and M241Pt model Pt-Ag (AgCl) electrode with the Honeywell model 31117481-501 reference electrode during the flotation experiment. Five minutes of conditioning time was targeted for the sulfidation. A Standard Hydrogen Electrode (SHE) was used for the pH measurement. Collectors were added stagewise during the flotation experiments. Moreover, three- and ten-minute conditioning times were applied for the collectors and depressants, respectively. The specifications of the reagents used in the study are shown in Table 1.
Table 1:Reagents used for the flotation experiments.
Result
Chemical and mineral composition
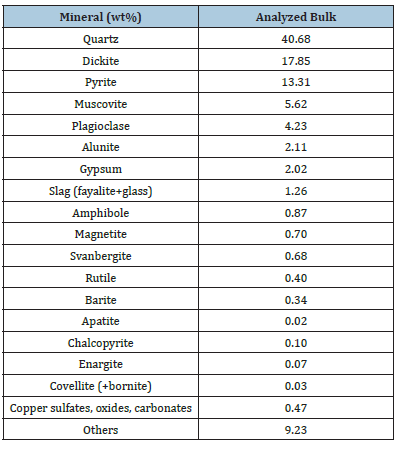
Based on the assays, the old Bor tailings sample contained 57.3% silica, 11.9% alu-mina, 7.7% iron, 7% sulfur, 0.24% copper, 0.41ppm gold, and 2.2ppm silver. Water and acid-soluble copper minerals carried almost 60% of the total copper within the sample. P1 refers to H2O dissolution, P2 to H2SO4 dissolution, P3 to KCN dissolution, and P4 to HNO3sub>+Br2 dissolution. The detailed chemical analysis is shown in Table 2. The main minerals of the sample were quartz (40.7 wt%), dickite (17.85 wt%), and pyrite (13.3 wt%).
The detailed mineralogy of the sample is presented in Table 3. The mineralogy of the old Bor tailings is complex due to the subsequent alteration of the primary sulfides and the occurrence of subsequent water- and acid-soluble copper minerals, like chalcanthite and different types of copper oxides. Copper in the old Bor tailings was carried by secondary chalcanthite-type minerals (57.5%) and by relicts of primary chalcopyrite (16.6%), enargite (16.3%), and covellite (9.6%). In addition, over 95% of the total sulfur content was carried in the pyrite. The sulfide fraction consisted of pyrite (95%), and the rest, 5%, included various copper sulfides and sulfates. The copper content of the sulfide fraction was 1.58%.
Table 2:Chemical composition of the feed sample.
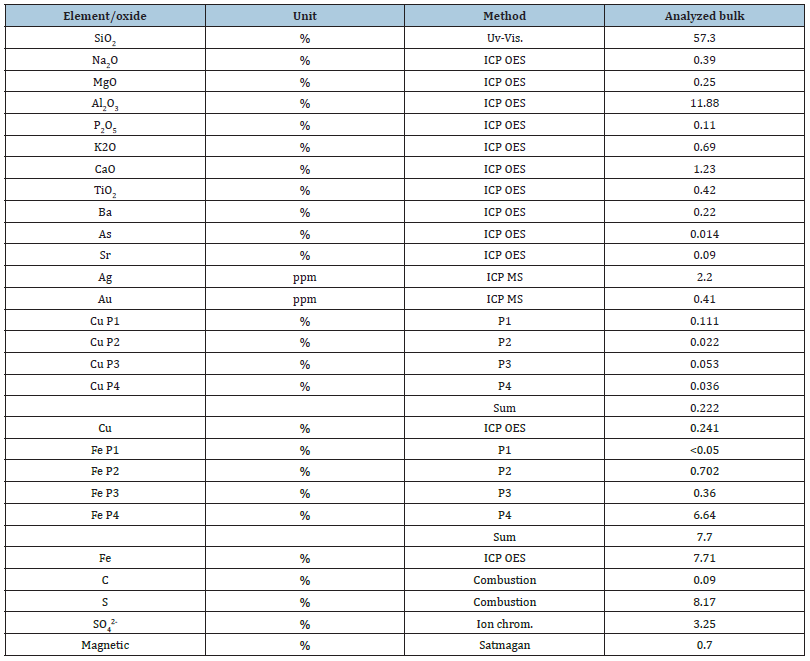
Table 3:Mineral composition of the analyzed bulk sample.
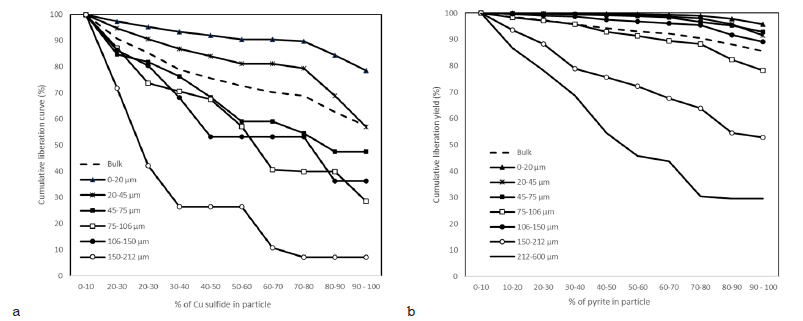
Based on the size-by-size liberation measurements, the copper minerals formed locked particles with gangue silicates and pyrite. In the coarser size fractions, copper minerals occurred as fine-grained inclusions in gangue silicates, and the appropriate liberation degree was reached in the finest -20μm size fraction. The bulk liberation degree of the copper minerals was 57%, i.e., 57% of the copper minerals were 90% to 100% liberated. Overall, 85.5% of the pyrite occurred as liberated grains, and the rest formed locked particles with silicates (Figure 5).
Figure 5:Cumulative liberation yield graphs for Cu sulfides (a) and pyrite (b).
Flotation experiments
Altogether 24 flotation experiments were carried out at a medium alkaline pH of 9.75-9.85 to prevent copper dissolution into the water phase by adding Ca(OH)2. Moreover, the dissolved copper was precipitated by adjusting the pH to >8. In the base case, Sodium Isobutyl Xanthate (SIBX), which has earlier been reported to be the most effective collector under medium alkaline conditions [12], was the primary collector, and polypropylene glycol methyl ether (Dow froth 250) was the main frother. Based on previous studies, the reagent dosages were 140g/t SIBX and 80g/t Dow froth 250.
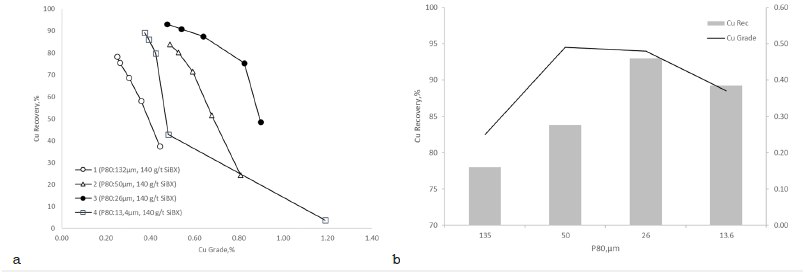
Effect of particle size on bulk flotation: To study the effect of different particle sizes on flotation experiments, the sample was ground for different grinding times to produce a particle size distribution (P80) between 50μm and 13.4μm. The highest copper grade, 0.49% Cu, was achieved when the P80 was 50μm. Although the highest copper recovery of 93% was obtained with a finer particle size having a P80 of 26μm, the concentrate grade, 0.46% Cu, remained slightly lower. The lowest copper recovery (78%) and grade (0.25% Cu) for the bulk concentrate were obtained without grinding at 132μm (P80). Decreasing the particle size of the feed improved both recovery and grade as the degree of liberation of sulfides increased (Figure 6).
Figure 6:Effect of particle size distribution on flotation performance a) copper grade versus copper recovery and b) bulk copper recovery and grade by P80.
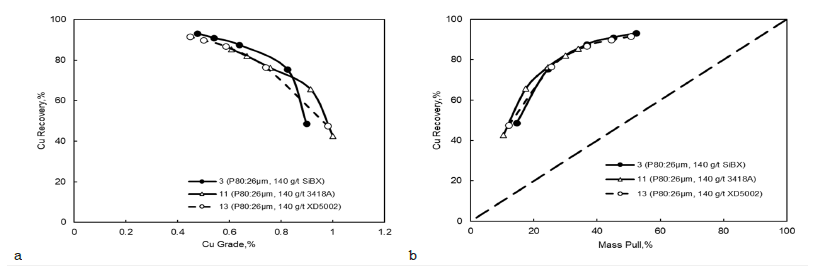
Effect of different collectors on bulk flotation: Thiocarbamate and dithiophosphates are some of the most important collectors that enable robust flotation performance, especially for oxidized copper minerals like those found in the Bor tailings. Therefore, Solvay’s 3418A (Sodium diisobutyl-dithiophosphinate) and XD 5002 (alkyl thiocarbamate) were tested to achieve better flotation performance [20]. According to the copper recoveries and grades, the overall flotation kinetics are al-most identical for both collectors. The different types of collectors did not improve the flotation performance and selectivity (Figure 7).
Figure 7:Effect of different promoters on bulk flotation performance a) copper grade versus copper recovery and b) copper recovery against mass pull on the concentrate..
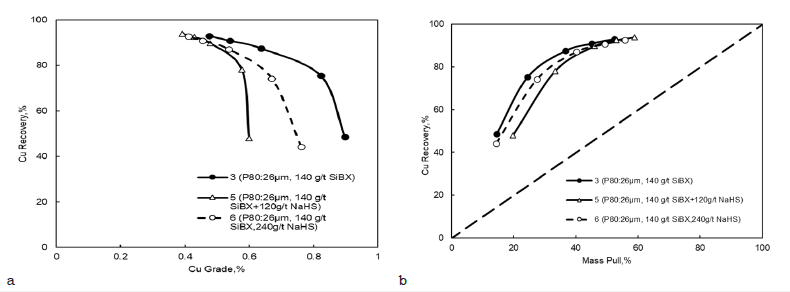
Effect of NaHS dosage: In the presence of oxidized minerals, sulfurizing agents such as sodium sulfide (Na2S) and sodium hydrosulfide (NaHS) are among the most common modifiers that increase the recovery of oxidized minerals [31]. The mineral surface of heavily oxidized sulfide minerals can be transformed into a hydrophobic surface by flotation modifiers such as NaHS. Therefore, NaHS makes such oxidized minerals more floatable in the presence of xanthate or thiol collectors [32].
The copper phase of the Bor tailings consists of oxide-type minerals. The pulp potential needs to be precisely adjusted to avoid excessive dosages using the sulfidation method. The flotation kinetic rate was increased by reducing the pulp potential using NaHS, but it also had a negative effect on the copper grades and recoveries. Additionally, when decreasing the pulp potential to below -150mV (Pt-Ag/AgCl) with NaHS, the flotation selectivity for gangue minerals decreased (Figure 8). Therefore, experiment number 3 with the reagent regimes 140g/t SIBX and 80g/t was determined as the baseline for the bulk stage.
Figure 8:Effect of NaHS dosage on bulk flotation performance a) copper grade versus copper recovery and b) copper recovery against mass pull.
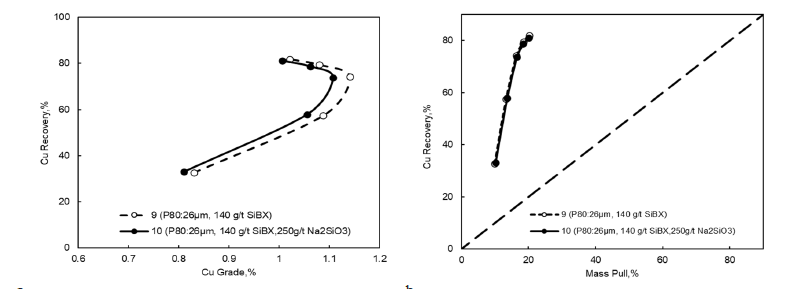
Effect of Na2SiO3 on cleaner stages: Test number 3 bulk flotation experiment conditions were used to produce material for the cleaner kinetic flotation experiments. All reagents were added stagewise in the cleaner flotation experiments to avoid excessive dosages and obtain proper kinetic trends. According to the results, an addition of 250g/t Na2SiO3 (Zeopol 30) did not improve the copper grade in the first cleaner concentrate. Based on the results, adding Na2SiO3 to the first cleaner stage had no major effect; therefore, in test number 9, cleaner flotation experiment conditions were selected (Figure 9).
Figure 9:Na2SiO3 on cleaner flotation performance a) copper recovery versus copper grade and b) copper recovery against mass pull.
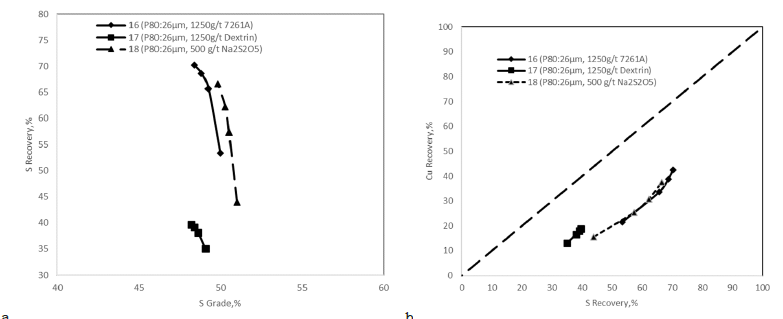
Copper-pyrite selective flotation: After determining the rougher and scavenger reagent conditions and the first cleaner flotation stage, a bulk concentrate was produced at 1% copper grade with 85% recovery. In the copper-pyrite selective flotation experiments, the aim was to depress pyrite in the presence of dextrin, polyacrylamide, and sodium metabisulfite. The depression mechanism of dextrin and polyacrylamide can be induced through interaction with surface ferric hydroxide on the pyrite. At the same time, the xanthate is adsorbed as dixanthogen to the surface ferric hydroxide [33,34]. On the other hand, in the presence of Na2S2O5, sulfide ions can be easily transformed to SO5-2 and these complexes can convert the copper sulfides on pyrite surfaces to copper oxides. Due to the oxidation of pyrite surfaces, xanthate collectors cannot attach to the pyrite surfaces, so pyrite is depressed [35,36] and copper minerals can be separated from the pyrite successfully.
Copper-pyrite selective flotation experiments on the second bulk cleaner concentrate were carried out by increasing the pH to 12. Moreover, 1250g/t polyacrylamide (Solvay 7261A), 1250g/t potato-based Dextrin, and 500g/t Na2S2O5 were used to depress the pyrite and float the copper minerals. The copper-pyrite selective flotation experiment results showed that different types of depressants did not depress pyrite in the copper circuit. However, 1250g/t of dextrin decreased the sulfur recovery to below 40% in copper concentrate. A decent quality (Cu>15%) of copper concentrate was not produced even though dextrin depressed pyrite in the copper-pyrite selective circuit (Figure 10). Therefore, the studied flowsheet focused on bulk flotation approach including the two-cleaner stages.
Figure 10:Effect of depressants on selective copper-pyrite flotation a) sulfur grade versus sulfur recovery and b) sulfur recovery versus copper recovery.
Flowsheet simulation: A simulation was carried out using HSC Chemistry ® 10 software on the preliminary flowsheet that was developed, including rougher, scavenger, and two bulk cleaner stages to determine the final copper and sulfur recoveries in a continuous process [28].
The HSC Sim Model Fit® tool was used for mass balance and middling stream calculation. In addition, the Klimpel flotation model for batch flotation was used to calculate kinetic parameters such as the infinitive recovery and the flotation rate constant. Mass balances were calculated, including grades and recoveries for each mineral in each stream. In the Klimpel model (Equation 1), Rmax shows the highest possible recovery for each mineral, and the k of rectangular distribution reveals how fast each mineral is floated (Metso, 2021):
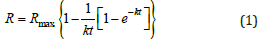
where t is the cumulative residence time, R max is the infinitive recovery, Kmax is the flotation rate constant, and Rmax≤1.
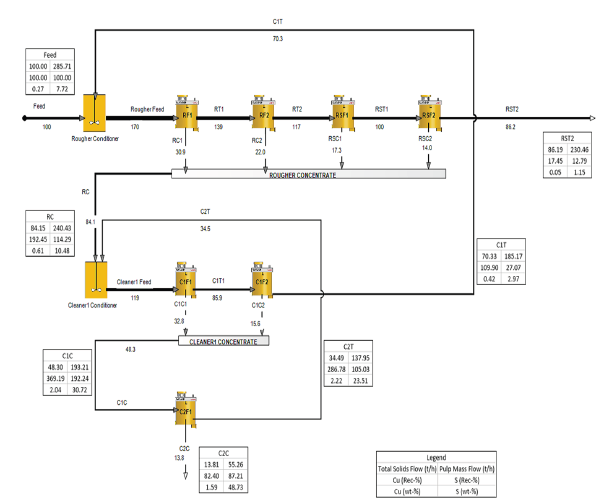
HSC Sim Model Fit® was used to draw kinetic curves for each mineral and define kmax and Rinf. These values were then used for flotation modeling. A tentative 100t/h plant capacity was selected and simulated using the rectangular distribution method for batch flotation time with Rmax and kmax constants. The simulation model calculated the residence time based on the volumetric pulp feed to the flotation bank with a fixed gas hold-up of 10%. The simulation model consisted of a 2x100m3 Metso Tank CellTM rougher and a 2x100m3 Metso Tank CellTM scavenger stage. Additionally, the first cleaner and second cleaner cell configurations were deter-mined as a 2x100m3 Metso Tank CellTM circuit and a 1x10m3 Tank CellTM, respectively. The solids content of the rougher and cleaner stages was adjusted to 35% w/w and 25% w/w, respectively.
Identifying the appropriate scale-up factor of geo metallurgically challenging tailings is always problematic due to the slower flotation kinetics and low-grade upgrade. Moreover, determination of scale-up factor is always a complex process which cannot be completely solved and usually results in the over scaling of laboratory data values [37]. Therefore, the scale-up factor was selected as 2.5 for this specific sample, taken from the Metso Research Center´s database system. According to the simulation results, a pyrite concentrate containing 48.73% S can be produced at 87% recovery. The pyrite concentrate contains 1.59% Cu at 82% recovery (Figure 11).
Figure 11:Simulated flowsheet with copper and sulfur grades and recoveries.
Conclusion
The main objective of the test work carried out by Metso was to show how valuables can be recovered from chemically and mineralogically challenging tailings. The Bor tailings contain 0.24% copper, 0.41ppm gold and 2.2ppm silver. Based on detailed chemical and mineralogical characterization, the main sulfide in the tailings is pyrite with accessory chalcopyrite, enargite, covellite and chalcanthite. Half of the total copper content is water and acid soluble. In the laboratory scale flotation tests, the focus is on evaluating the effect of particle size, different types of collectors, pH, and pulp potential on achieved grades and recoveries. The dissolution of copper can be prevented by adjusting pH and Eh. Based on the test work conducted, both copper and gold can be recovered effectively into pyrite concentrate [38,39].
Finally, 85% of sulfur and 80% of copper were recovered in into concentrate. The recoveries for gold and silver were 49% and 84%, corresponding. The final concentrate contained 50.7% sulfur, 1.65% copper and 9ppm gold. Reprocessing of old mine tailings can be both economic and environmentally friendly solution through recovering a substantial amount of copper, gold, and sulfur into concentrate, that can be utilized in smelting as a source of energy or fed to sulfuric acid plants. Simultaneously, the reprocessing helps minimize the environmental impact associated with acid mine drainage [40,41].
Author Contributions
Conceptualization: JL, AL, and EÖ; methodology: EÖ and AL; resources: JS; writing-original draft preparation: JL; writing-review and editing: JL, E.Ö, AL, and JS; supervision: JS. All authors have read and agreed to the published version of the manuscript.
Funding
This research has received funding from the European Institute of Innovation and Technology (EIT). This body of the European Union receives support from the European Union’s Horizon 2020 research and innovation program.
Acknowledgment
We would like to acknowledge the anonymous reviewers for their constructive comments and valuable insight. The laboratory technicians at Metso Outotec Research Center are appreciated for their competent assistance in the experimental and analytical work. Mr. Matthew Hicks and Dr. Ahmad Mardoukhi are thanked for their valuable comments. Thanks, are also due to Mr. Mike Jones and Ms. Sue Pearson from Pelc Southbank Languages for correcting the English manuscript.
References
- Xie Y, Xu Y, Yan L, Yang R (2005) Recovery of nickel, copper and cobalt from low-grade Ni-Cu sulfide tailings. Hydrometallurgy 80(1-2): 54-58.
- Lutandula MS, Maloba B (2013) Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidized ores. Journal of Environmental Chemical Engineering 1(4): 1085-1090.
- Falagán C, Grail BM, Johnson DB (2017) New approaches for extracting and recovering metals from mine tailings. Minerals Engineering 106: 71-78.
- Babel B, Penz M, Schach S, Boehme S, Rudolph M (2018) Reprocessing of a Southern Chilean Zn tailing by flotation-A case study. Minerals 8: 295.
- Drobe M, Haubrich F, Gajardo M, Marbler H (2021) Processing tests, adjusted cost models and the economies of repro-cessing copper mine tailings in Chile. Metals 11(1): 103.
- Shengo LM (2021) Potentially exploitable reprocessing routes for recovering copper and cobalt retained in flotation tailings. Journal of Sustainable Metallurgy 7(1): 60-77.
- Armstrong R, Kozelj D, Herrington R (2005) The Majdanpek Cu-Au deposit of Eastern Serbia, a review. In: Porter TM (Ed.), Super porphyry copper and gold deposits-A global perspective: Adelaide, PGC Publishing 2: 453-466.
- Antonijević I, Mijatović P (2014) The copper deposits of Bor, eastern Serbia: Geology and origin of the deposits. Geological Annals of the Balkan Peninsula 75: 59-74.
- EJATLAS; Environmental Justice Atlas: Over a century of pollution from the Bor mines, Serbia.
- Urošević S, Vuković M, Pejčić B, Štrbac N (2018) Mining-metallurgical sources of pollution in Eastern Serbia and environmental consciousness. International Journal of Environmental Pollution 34(1): 103-115.
- Antonijević MM, Dimitrijević MD, Stevanović ZO, Serbula SM, Bogdanovic GD (2008) Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. Journal of Hazardous Materials 158(1): 23-34.
- Markovic Z, Vusovic N, Milanovic D (2010) Old copper flotation tailings waste processing. Proceedings of XXV International Mineral Processing Congress (IMPC). Brisbane, QLD, Australia, pp. 3825-2829.
- Jambor JL (1994) Mineralogy of sulfide-rich tailings and their oxidation products. In Short course handbook on environmental geochemistry of sulfide mine-wastes. Mineralogical Association of Canada, Nepean, Canada.
- Đorđievski S, Ishiyama D, Ogawa Y, Stevanović Z (2018) Mobility and natural attenuation of metals and arsenic in acidic waters of the drainage system of Timok River from Bor copper mines (Serbia) to Danube River. Environmental Science and Pollution Research 25(25): 25005-25019.
- Antonijević MM, Dimitrijević MD, Milić SM, Nujkić MM (2012) Metal concentrations in the soils and native plants sur-rounding the old flotation tailings pond of the copper mining and smelting complex Bor (Serbia). Journal of Environmental Monitoring 14(3): 866-877.
- Filimon MN, Caraba IV, Popescu R, Dumitrescu G, Verdes D, et al. (2021) Potential eco-logical and human health risks of heavy metals in soils in selected copper mining areas-a case study: The Bor area. International Journal of Environmental Research and Public Health 18(4): 1516.
- Stanković V, Milošević V, Milićević D, Gorgievski M, Bogdanović G (2018) Reprocessing of the old flotation tailings deposited on the RTB Bor tailings pond-a case study. Chemical Industry and Chemical Engineering Quarterly 24(4): 333-344.
- Conić V, Stanković S, Marković B, Božić D, Stojanović J, et al. (2020) Investigation of the optimal technology for copper leaching from old flotation tailings of the copper mine Bor (Serbia). Metallurgical and Materials Engineering 26(2): 209-222.
- Stanojlović RD, Sokolović JM, Milosević N (2014) Integrated environmental protection and waste minimization in the area of Copper Mine Bor, Serbia. Environmental Engineering & Management Journal (EEMJ) 13(4).
- Bulatovic SM (2007) Handbook of flotation reagents: chemistry, theory and practice: flotation of sulfide ores. Elsevier, pp. 276-277.
- Han B, Altansukh B, Haga K, Stevanovic Z, Radojka J, et al. (2014) Copper upgrading and recovery process from mine tailing of Bor region, Serbia using flotation. International Journal of the Society of Materials Engineering for Resources 20(2): 225-229.
- Leppinen J, Salonsaari P, Palosaari V (1997) Flotation in acid mine drainage control: beneficiation of concentrate. Canadian metallurgical Quarterly 36(4): 225-230.
- Wang L, Liu R, Hu Y, Liu J, Sun W (2016) Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technology 302: 15-20.
- Rao DS, Vijaya Kumar TV, Rao SS, Prabhakar S, Raju GB (2011) Effectiveness of sodium silicate as gangue depressants in iron ore slimes flotation. Int J Miner Metall Mater 18: 515-522.
- Serafimovski T, Ristovic I, Boev B, Tasev G, Boev I, et al. (2021) Mineralogical analysis of samples from the old Bor mine flotation tailing, Republic Serbia. Natural Resources and Technologies 15(1): 37-50.
- (2023) RIS-CuRE project.
- Young RS (1974) Chemical Phase Analysis. London.
- Lamberg P, Hautala P, Sotka P, Saavalainen S (1997) Mineralogical balances by dissolution methodology. Proceedings of Short Course on Crystal Growth in Earth Sciences, Mamede de Infesta. International Mineralogical Association pp. 1-29.
- Lund C, Lamberg P, Lindberg T (2013) Practical way to quantify minerals from chemical assays at Malmberget iron ore operations–An important tool for the geo metallurgical program. Minerals Engineering 49: 7-16.
- Metso (2023) Outotec HSC Chemistry Software.
- Yin W, Sun Q, Li D, Tang Y, Fu Y, et al. (2019) Mechanism and application on sulphidizing flotation of copper oxide with combined collectors. Transactions of Nonferrous Metals Society of China 29(1):178-185.
- Leja J (2007) Surface chemistry of froth flotation. Quebec, Springer. pp:197-255.
- López-Valdivieso A, Sanchez-Lopez AA, Padilla-Ortega E, Robledo-Cabrera A, Gálvez ED, et al. (2018) Pyrite depression by dextrin in flotation with xanthates. Adsorption and floatability studies. Physicochemical Problems of Mineral Processing 54: 1159-1171.
- Yang X, Mu Y, Peng Y (2021) Comparing lead and copper activation on pyrite with different degrees of surface oxidation. Minerals Engineering 168: 106926.
- Chandra AP, Puskar L, Simpson DJ, Gerson AR (2012) Copper and xanthate adsorption onto pyrite surfaces: Implications for mineral separation through flotation. International Journal of Mineral Processing 114-117(21): 16-26.
- Chen X, Peng Y, Bradshaw D (2013) Effect of regrinding conditions on pyrite flotation in the presence of copper ions. Inter-national Journal of Mineral Processing 125: 129-136.
- Diego M, Pablo B (2019) Scale-up in froth flotation: A state-of-the-art review. Separation and Purification Technology 210: 950-962.
- Alexander C, Johto H, Lindgren M, Pesonen L, Roine A (2021) Comparison of environmental performance of modern copper smelting technologies. Cleaner Environmental Systems 3: 100052.
- Bryk P, Ryselin J, Honkasalo J, Malmstrom R (1958) Flash smelting copper concentrates. JOM 10(6): 395-400.
- Ejtemaei M, Nguyen A (2017) Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Minerals Engineering 100: 223-232.
- Kojo IV, Storch H (2006) Copper production with Outokumpu flash smelting: An update. Advanced Processing of Metals and Materials pp. 226-238.
© 2023 Erdem Özdemir. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






