- Submissions

Full Text
Aspects in Mining & Mineral Science
A Moissanite-Diamond-Graphite Paragenesis in a Small Beryl-Quartz Vein Related to the Variscan Tin-Mineralization of the Ehrenfriedersdorf Deposit, Germany
Rainer Thomas1*, Ulrich Recknagel2 and Adolf Rericha3
1Im Waldwinkel 8, D-14662 Friesack, Germany
2Böhmerwaldstraße 22, D-86529 Schrobenhausen, Germany
3Alemannenstraße 4a, D-144612 Falkensee, Germany
*Corresponding author:Rainer Thomas, Im Waldwinkel 8, D-14662 Friesack, Germany
Submission: July 13, 2023: Published: July 26, 2023

ISSN 2578-0255Volume11 Issue5
Abstract
After the proof of ultrahigh-pressure mineral inclusions in the Variscan Greifenstein granite, we looked for further hints of supercritical indications in the granite-related tin mineralization of Ehrenfriedersdorf, German Erzgebirge. Here we found an unusual moissanite-diamond-graphite paragenesis in a small beryl-quartz vein. That is the first found of SiC and diamond grown in a granite-related pegmatite-hydrothermal system under a low-pressure regime (≤ 2kbar). The study of rare melt inclusions showed a crystallization temperature of 700-720 °C for the simultaneous growth of beryl and moissanite. Raman spectroscopy was the primary method for identifying moissanite, nanodiamond, and graphite. According to these measurements, the SiC crystals and needles are mainly of the hexagonal 6H-SiC polytype and twisted along the c-axis. Moissanite in beryl generally forms needle- or whisker-like crystals parallel to the c-axis of beryl. Nanodiamond crystals are directly in the moissanite, or the moissanite-graphite matrix distributed, showing a clear crystallographic relationship to the beryl substrate. Besides the whisker-like moissanite crystals in beryl grown by two different mechanisms, there are also more isomorphic and spheric moissanite crystals in beryl and quartz. Up to now, the growth mechanism of the whisker-like crystals is not precisely known. Two mechanisms compete for a natural supercritical Vapor-Liquid-Solid method (VLS) and a low-pressure heteroepitaxial Chemical Vapor Deposition process (CVD).
Keywords:Moissanite; Nanodiamond; Graphite; Beryl; Quartz; Raman spectroscopy; Supercritical fluids
Introduction
In several papers, the author has demonstrated [1-4] that supercritical fluid has a more significant meaning for the enrichment and re-distribution of elements than generally assumed. By the specific properties of such fluids (density between 100 and 1000kg/m3, viscosity between 50-100μPa.s, and diffusivity between 0.01 and 0.1mm2/s - see [5]) and the high velocity, they can also transport small crystalline particles from the origin depths as we have shown. To such belong spherical/elliptical crystals of stishovite, coesite, zircon-reidite, and many others [4]. Up to now, it was, however, unclear which chemical character such melts or fluids have. A new impulse came from finding ultra-high-pressure minerals in the Greifenstein granite near Ehrenfriedersdorf [6]. Sensitive by clear proof of moissanite and diamond in this granite as foreign bodies (not remnants of abrasives discussed by Keller & Ague [7]), the authors found new essential hints to the character of such fluids. In this paper, we briefly show the most important results. A crucial experience was finding moissanite and diamond in some beryl-bearing vein samples from the Sauberg mine near Ehrenfriedersdorf [8]. In difference to the moissanite and diamond in the Greifenstein granite [6], moissanite, diamond, and beryl are genetically directly linked. Of course, a couple of papers describe the shallow origin of diamonds [9,10] and a scathing reply to such shallow origin by Yang et al.[11]. However, the origin of these ophiolite-related diamonds has nothing to do with the case described here. Another interpretation gives El Mendili et al. [12]. According to these authors, diamond and moissanite polytypes are inherited from serpentinized peridotites crystallized in the lower mantle.
Sample Material
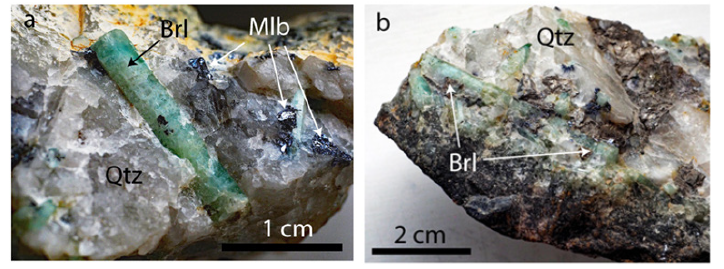
The beryl-quartz sample material comes from the Sauberg mine near Ehrenfriedersdorf in the Erzgebirge region/Germany. Figure 1 shows the location of this deposit around Ehrenfriedersdorf. Also shown is the Greifenstein granite, where we found UHP mineral inclusions in the crustal granite for the first time [6]. Schröcke [13] and Hösel [14] describe impressive the Variscan tin deposits around Ehrenfriedersdorf. However, a description of the small beryl veins is missing. Only Kumann & Leeder [15] discuss this very shortly in their contribution and show such veins’ appearance. The sample is from a 6cm thick beryl-bearing quartz veinlet (composed of 28% beryl, 72% quartz) from the Sauberg mine (Prinzler Liegendtrum, samples TU BAF F64-18). The pieces (Figures 2a & 2b) are composed mainly of about 3x0.5cm large green beryl in a weak smoky-colored quartz matrix, and this quartz related to beryl is a little bit older [8]. The vapor phase of the primary fluid inclusions in quartz and beryl is mainly CH4. Samples from smaller veins are composed nearby only of beryl (90%) and minor quartz. The adjacent rock is greisen. For the microscopic and Raman spectroscopic studies, we used double-polished quartz-beryl chips with a thickness of 500μm. The samples were never coated with carbon for electron microprobe studies.
Figure 1:Schematic geological map of the deposit district of Ehrenfriedersdorf, German Erzgebirge (according to Rösler et al. [34]). Legend: 1-phyllite, 2-mica shist, 3-red gneiss, 4-dark mica shist, 5-biotite-muscovite shist, 6-gneiss, 7-augengneiss, 8-amphibolite shist, 9-limestone, 10-skarn, 11-granite, 12-Sn lodes, 13-Bi-Co-Ni lodes.
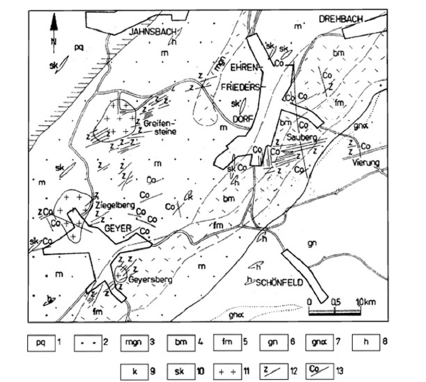
Figure 2a-2b:Details of the studied quartz-beryl sample from the Sauberg mine near Ehrenfriedersdorf: Brl - green beryl, Mlb-Molybdenite, Qtz-Quartz.
In the beryl and quartz, there are tiny nearby colorless elliptical moissanite, beryl-moissanite, quartz-cristobalite, and zirconreidite crystals for which Thomas et al. [4] offered an interpretation as indications of fast upward moving supercritical fluid. Figure 3a & 3b shows spherical inclusions of moissanite and beryl-moissanite intergrowth in quartz. The beryl of the beryl-moissanite intergrowth (Figure 3b) shows an extensive formation of twinnings, which may form at a pressure of about 14GPa [16]. In addition, many spherical to oval aggregates composed of phosphates like jahnsite-(CaMnFe), nearby colorless sphalerite [ZnS], polycrystalline, and amorphous graphite are present. Separate beryl crystals in the quartz matrix often show a rim of carbonic material. The dark sample walls (at the border to the adjacent rock) contain cassiterite, arsenopyrite, molybdenite, muscovite, sphalerite, trilithionite, and many minor ore minerals. The beryl is transparent and emerald to pale green and is rich in fluid inclusions, generally of secondary or pseudosecondary character. Rarer are water-rich silicate melt inclusions of a primary nature. Thomas et al. [17] state that these melt inclusions homogenize between 700-720 °C near the solvus crest for this sample. The pressure is ≤ 2kbar [18]. The sample material was given by Prof. L. Baumann from the Mining Academy Freiberg in 1980 as reference material.
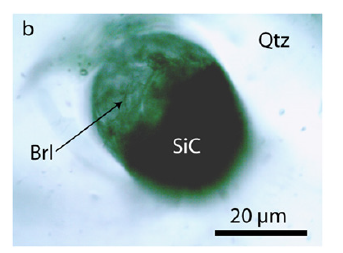
Figure 3a:Moissanite (SiC) inclusion in quartz (Qtz) depth under the surface, also indicated by intact fluid inclusions.
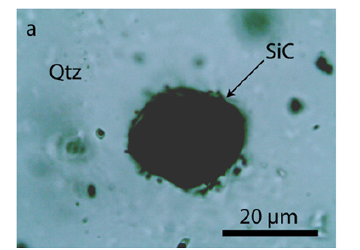
Figure 3b:Spherical crystal of beryl (Brl) and moissanite (SiC) in quartz (Qtz). Beryll was primarily beryl-II - the high-pressure phase of beryl [16].
Methodology
We have performed all microscopic studies with a petrographic polarizing microscope with a rotating stage coupled with the RamMics R532. Raman spectrometer. The Raman spectra were recorded in the spectral range of 0-4000cm-1 using a 50mW singlemode 532nm laser, entrance aperture of 20μm, holographic grating of 1800g/mm, and spectral resolution ranging from 4-6cm-1. We used microscope objective lenses with a magnification varying from 3.2x to 100x depending on the grain size. We used the Olympus longdistance LMPLFLN100x for most measurements as a 100x objective lens. The laser energy on the sample is adjustable down to 0.02mW. The Raman band positions were calibrated before and after each series of measurements using the Si band of semiconductor-grade silicon single-crystal. We used a water-clear natural diamond crystal as a diamond reference (for more information, see [2]). For the fast mineral identification with the Raman-micro-spectroscopy, we used the RRUFF database and the CrystalSleuth program [19]. Note here that the Raman spectrometry is, according to Tuschel [20], the most efficacious method for characterizing, differentiation, and screening polymorphs (here, SiC). Furthermore, the polarization/ orientation (P/O) micro-Raman spectroscopy is complementary to micro-X-ray diffraction. According to Tuschel [21], this method should use when X-ray analysis is not practical or possible (microneedles and mineral globules depth in the sample volume).
Result
Figure 4:Beryl aggregate with many needle-like moissanite crystals. Nearby all black parts in this photomicrograph are moissanite.
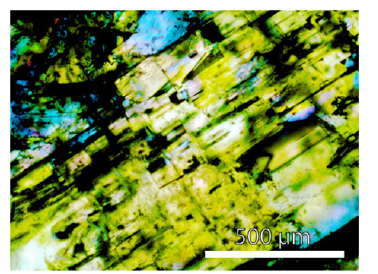
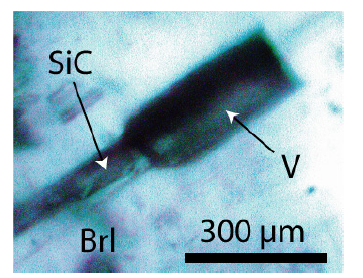
The Raman spectrum of the studied beryl shows the typical very strong bands at 684 and 1068cm-1 and the medium to strong bands at 320.9, 395, and 1912cm-1. The beryl of the samples contains many black, often spherical aggregates of moissanite, graphite, and diamond (with an average of about 15000 to 35000 SiC crystals per 1cm3 of the sample volume; and in rare single beryl crystal aggregates the maximum is 280000 SiC crystals/ cm3 - see Figure 4). SiC sheathes nanodiamonds, mostly. From microscopic and Raman spectroscopic studies, it is evident that the SiC-diamond aggregates are not the result of preparation (see also [4]). The morphology and the Raman band position generally differ from SiC used for grinding. Many larger aggregates (~75x200μm) are more rectangular or spherical. Especially interesting are thin moissanite needles parallel to the beryl c-axis with dimensions of about 10x10x600μm (Figures 5a-5d). Often the needles are twisted along the c-axis. Up to now, the most extended needle is 5200μm long and 60 to 80μm thick. The cross-section is circular to elliptical (dependent on the cutting slope), and sometimes there are two or more needles in one beryl crystal (Figure 6).
Figure 5:a) A moissanite (SiC) needle in the center of a beryl (Brl) crystal. The needle is not at the beryl surface. b) The SiC-needle is 600μm long. c) the same moissanite needle at greater depth. Shown is also a pseudo primary type-B melt inclusion. The inserted photomicrograph show some details. The daughter minerals are silicates, and the gas phase consists of pure methane (CH4). d) Light-microscope image of a moissanite needle in beryl (Brl). SiCMoissanite, nD-Nanodiamond, Gr-Amorphous carbon.
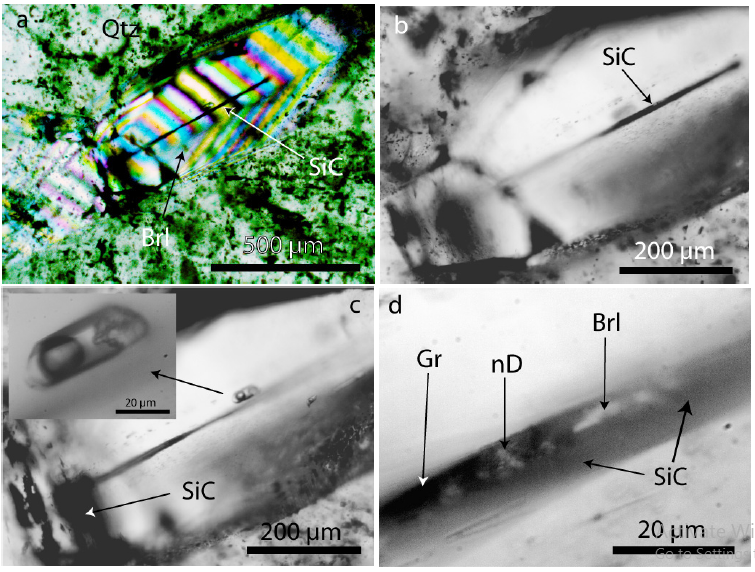
Figure 6:Several SiC whiskers parallel to the c-axis of a beryl crystal (Brl) from the Ehrenfriedersdorf Sauberg mine. Figures a) and b) show the same position in different depths. Figure 6c shows lancet-like moissanite crystals in beryl.
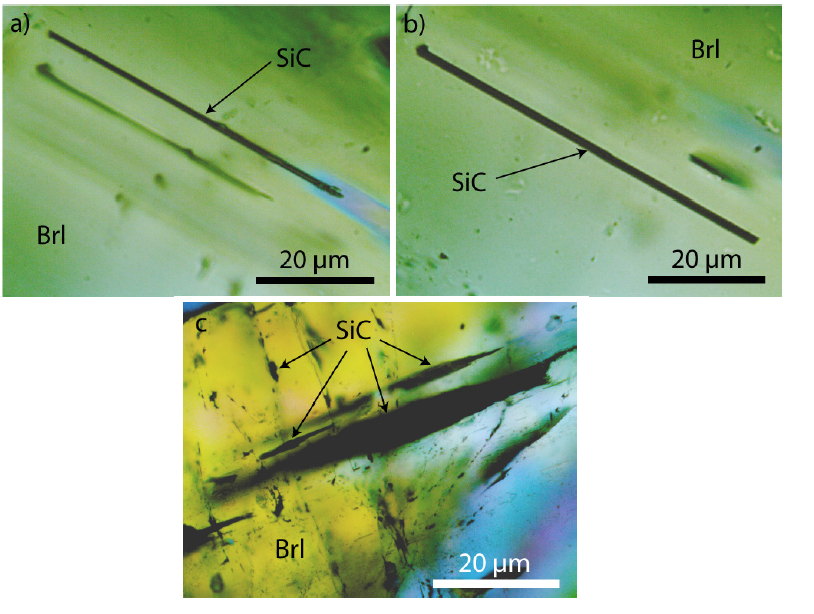
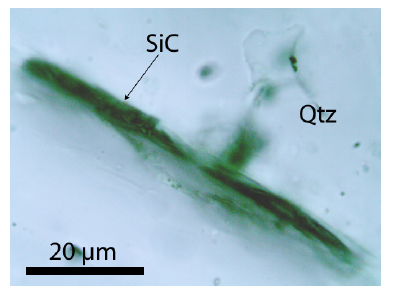
Rarer than moissanite crystals that have grown parallel to the c-axis are tiny moissanite needles perpendicular to the c-axis. Moissanite is if graphite is missing, transparent, and of light grey color. The whisker-like moissanite crystals obviously use the water-filled channels of beryl. The discovery of SiC needles in the beryl mineralization, a component of the Variscan tin deposit Ehrenfriedersdorf, is absolutely a novum. Because, in such low crustal depth (< 2kbar), nobody would expect the formation of moissanite. Because usually, the moissanite-diamond association is an ultra-high pressure phase. The same is valid for the diamond formation connected with moissanite crystallization because diamond forms, as a rule, inclusions (nanodiamonds) in moissanite. Free macroscopic diamond crystals in beryl are not present. Apart from the spherical intergrowth of beryl and moissanite in quartz (foreign body yielded by the supercritical fluid), however, as already said, moissanite is not only connected to beryl. The paragenetic quartz also contains whisker-like moissanite crystals (Figure 7). These rare cases show mostly signs of destruction, maybe caused by the α-β transformation of the high-quartz to the low-quartz.
Figure 7:Whisker-like moissanite crystal in quartz.
Raman spectroscopic results
Figure 8a presents a typical Raman spectrum of a SiC needle in beryl, and Figure 8b is a Raman spectrum of a 13C-enriched SiC crystal in the same sample [22]. The prominent Raman peak of SiC is at 768.0±8.3cm-1 with a linewidth (FWHM) of 30.8±4.6cm-1. Also, the second intense Raman band in the spectrum at 940.3±9.6cm- 1 shows a large linewidth of 48.3±13.7cm-1. According to the first measurements, the SiC needles are of the hexagonal 6H-SiC polytype. We have also found the cubic 3C-SiC and the orthorhombic 6O-SiC polytypes in larger aggregates. Table 1 shows the experimental determined Raman-active vibration modes of the predominant SiC polytype (6H-SiC) in beryl from Ehrenfriedersdorf. The Raman data for a reference sample of technical SiC (with its natural isotope abundance) are given for comparison.
Figure 8a:Typical Raman spectrum of a SiC needle in beryl. Brl-Beryl Raman bands.
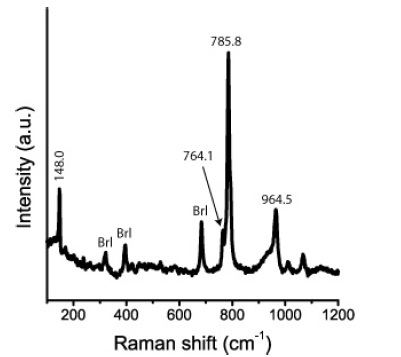
Figure 8b:Raman spectrum of a 13C-rich moissanite crystal in beryl [22].
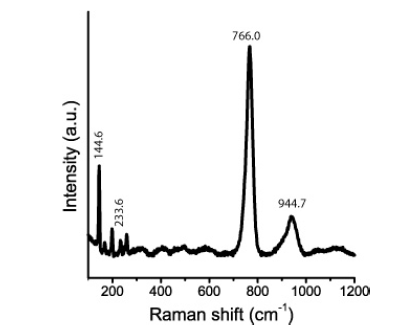
Table 1:Experimental Raman-active vibrational modes of the SiC polytype 6H-SiC in beryl from Ehrenfriedersdorf, Erzgebirge, Germany, and a synthetic/technical reference. n-Number of studied SiC crystals; vibration modes according to [6,7]: AA-Axial Acoustic Mode, LA-Longitudinal Acoustic Mode, LO-Longitudinal Optic Mode, PA-Planar Acoustic Mode, PO-Planar Optic Mode, TA-Transversal Acoustic Mode, TO-Transversal Optic Mode. Intensity: vw-very weak, w-weak, m-medium, s-strong, vs-very strong; FWHM-linewidths (Full Width at Half Maximum).
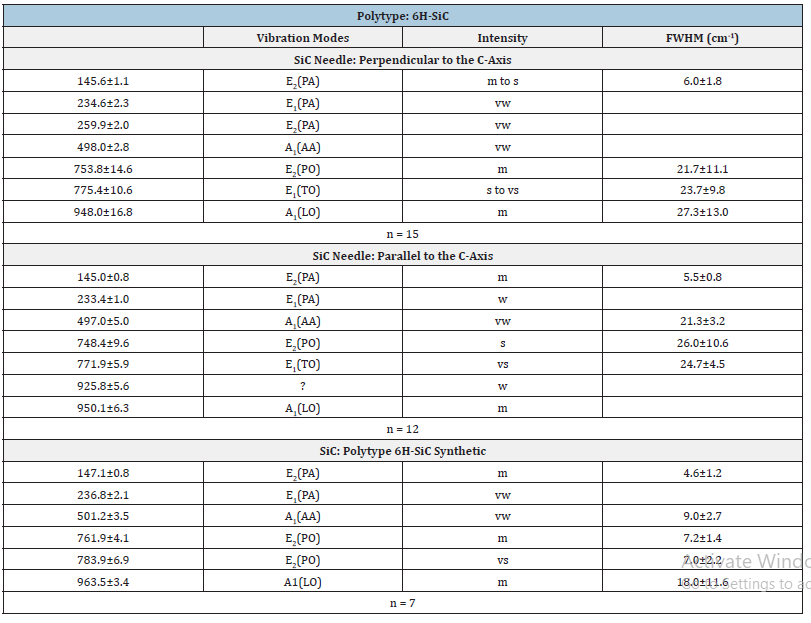
Table 2:Experimental Raman-active vibrational modes of the SiC polytype 6H-SiC in quartz from Ehrenfriedersdorf, Erzgebirge, Germany. n-number of studied SiC crystals; vibration modes according to [6,7]: AA-Axial Acoustic Mode, LA-Longitudinal Acoustic Mode, LO-Longitudinal Optic Mode, PA-Planar Acoustic Mode, PO-planar optic mode, TA - Transversal Acoustic Mode, TO-Transversal Optic Mode. Intensity: vw-very weak, w-weak, m-medium, s-strong, vs-very strong; FWHM-linewidths (Full Width at Half Maximum).
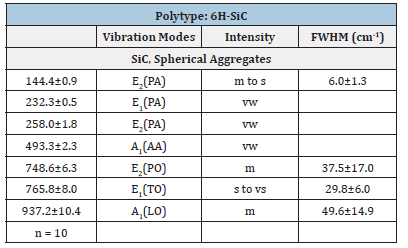
Table 3:Experimental Raman-active vibrational modes of 13C-rich 6H-SiC and 6O-SiC in beryl from Ehrenfriedersdorf, Erzgebirge, Germany. n-number of studied SiC crystals; vibration modes according to Chikvaidze et al. [27] and El Mendili et al. [28]: TA-Transversal Acoustic Mode, TOTransversal Optic Mode, LA-Longitudinal Acoustic Mode, LO-Longitudinal Optic Mode. Intensity: vw-very weak, w-weak, m-medium, s-strong, vs-very strong; FWHMlinewidths (Full Width at Half Maximum).
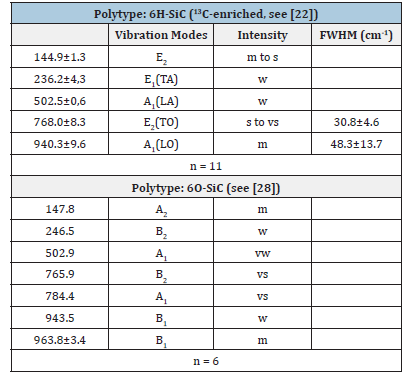
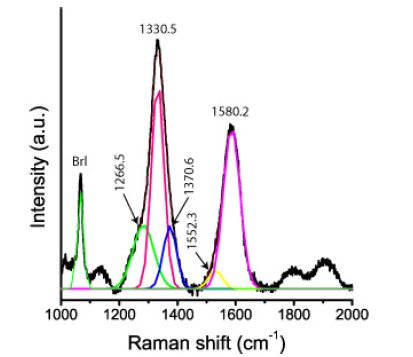
Table 2 gives the obtained Raman data for moissanite in the paragenetic quartz of the sample. The Raman data for the natural moissanite in beryl and quartz are similar; however, they distinguish significantly from the technical used SiC for preparation. For sample preparation, we used only SiC powder (F10 with a 10μm mean grain size). This technical-grade SiC is of 6H-SiC polytype and exhibits prominent bands at 784.0±3.6cm-1 and 963.1±2.4cm- 1 (Table 1 and [4]). More apparent are the differences between technical SiC and natural 13C-enriched moissanite in beryl (Table 3). Figure 9 shows a typical Raman spectrum of nanodiamonds in the beryl sample. The linewidth of this diamond is extremely large: 62.3cm-1 too. All nanodiamonds in six different beryl samples show such large line widths. The mean of the first-order band of diamond along the needle (Figure 5a) is 1328.6±5.6cm-1 (14 points), and the shoulder is 1268.1±13.1cm-1. This FWHM (60cm-1) and the shoulder at ~1260cm-1 speak for the formation of nanodiamonds [23,24] at the crystallization of moissanite together with beryl. Nanodiamonds from a second moissanite needle in a beryl crystal give 1322.8±5.5cm-1 and a linewidth of 61.4±18.9cm-1 (10 points along the SiC crystal). These values argue for the presence of lonsdaleite, the hexagonal diamond polytype.
Figure 9:Typical Raman spectrum of diamond in beryl. The FWHM of the diamond is 62.3cm-1. Brl-Beryl. The band at 1580cm-1 represents the G-band (the main Raman line of graphite). The colored curves show the mathematical deconvolution of the diamond-carbon spectrum into Lorentzian components.
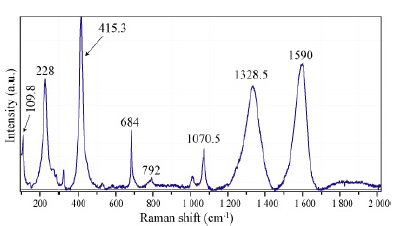
That nanodiamond is already present in the supercritical fluid, as follows from Raman spectrometric measurements on large primary fluid inclusions in beryl (Figure 10). Note here that such large inclusions are seldom. Most similar inclusions are significantly smaller, however widespread. The vapor phase consists exclusively of methane (CH4), and cristobalite (Crs) forms the solid phase of both inclusions. A necking-of process creates both inclusions [25]. Cristobalite in the methane inclusion is stable under laser light and is metastable in the water-bearing fluid inclusion (forming quartz). With the used Raman spectrometer, in no case hydrogen using the pure rotational lines S0 (354.8cm-1), S1 (587.4cm-1), S2 (815.0cm-1), and S3 (1024.9cm-1) could be determined - see [26]. Also, CO2 could not be determined. The cristobalite was primarily water-rich stishovite or coesite, giving the supercritical fluid a minimum pressure of 7GPa. Figure 11 shows a Raman spectrum of the cristobalite in the CH4-rich inclusion of Figure 10. The strong and stable nanodiamond band at 1328cm-1 and the strong carbon band at 1590cm-1 are conspicuous. All larger moissanite crystals show these characteristic Raman bands at about 1330 and 1580cm- 1 (Figure 9).
Figure 10:Two supercritical inclusions in beryl (Brl). CH4-Methane, Fl-Aqueous Liquids Phase. Cristobalite (Crs) in both inclusions gives in the Raman spectrum strong lines at 112.6, 229.8. 418.6, and 1072.1cm-1, respectively. Both inclusions result from a necking-of process [25].
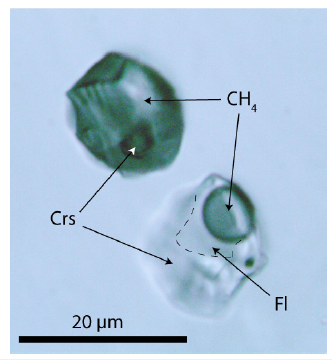
Figure 11:Raman spectrum of cristobalite, nanodiamond, and carbon in the CH4-rich inclusion of Figure 10. The Raman bands at 109.8, 228, 414.3,792, and 1070.5cm-1 correspond to cristobalite. The 1328.5 and 1590 cm-1 bands correspond to nanodiamonds and carbon, respectively.
Discussion
Moissanite comprises many SiC polytypes that crystallize in cubic, hexagonal, and rhombohedral symmetries [27,28]. A precise frequency distribution of the specific SiC polytypes of the found moissanite must remain for forthcoming studies. Also, 13C-12C isotope determination is necessary. However, the dominant polytype here in beryl is 6H-SiC. The finding of simultaneously grown moissanite in the beryl of a tin deposit formed at low pressure and temperature is astonishing. Moissanite is a rare mineral, forming inclusions in kimberlite diamonds or other ultramafic rocks, such as lamproites, under high pressures and temperatures. Here, moissanite forms large aggregates and crystal needles in beryl. Generally, moissanite is intergrown crystallographically with the beryl host. According to Schmidt et al. [29], highly fractionated ultra-reducing COH-fluids are necessary for forming moissanite. However, such fluids are uncommon for crustal appearance. Also, indications of the required Iron-Wüstite (IW) buffers are missing. A mantle-derived supercritical fluid [4] reacts obviously with graphite-rich metapelites [14] from the neighborhood, forming graphite-saturated CH4-H2-H2S-rich fluids [30]. Because there are some primary high-temperature (700- 750 °C) melt inclusions (with CH4 in the vapor phase) in the beryl sample [17], we can accept at the beginning temperatures around 750 to 800 °C for such reducing COH-fluids if they react with the granite-related mineralization. Higher primary temperatures (~1000 °C) for the supercritical fluid or melt are also possible, as indicated by the appearance of kumdykolite [NaAlSi3O8] (a hightemperature feldspar) in the Greifenstein granite’s topaz and parts of the late Ehrenfriedersdorf cassiterite mineralization [6]. This work expands the types of spheric crystals transported by the supercritical fluid by beryl, moissanite, and intergrowth of beryl with moissanite and nanodiamond. During the fast crystallization of beryl, these extraneous phases were trapped as such (without obtaining the equilibrium crystallographic form).
Looking at the Raman data in Table 1, it is conspicuous that the prominent Raman bands show a relatively significant shift (~20-25cm-1) to lower values. According to Raman measurements on isotopic characterized SiC samples (by secondary ion mass spectrometry SIMS), Ivanov et al. [22] interpreted such a shift as a significant increase in the 13C concentration. 20cm-1 corresponds to a rise of about 40%, which means a selective 13C incorporation during the growth of SiC during the beryl crystallization. Such a shift is also a strong argument for the natural origin of the moissanite in beryl. Figure 11 shows the omnipresent Raman doublet at about 1330 and 1590cm-1 beside the cristobalite bands at about 230 and 415cm-1. This doublet is present in all larger moissanite crystals (whisker, spherical, and more isometric crystals). In the beryl part of the globule (Figure 3b), there are beside the nanodiamond and the strong carbon G band at 1580cm-1 in the high-frequency range two bands at 2851 and 2883cm-1 present. According to Buchner et al. [31] these bands represent CHX groups on the surface of nanodiamonds. Similar results came already from Frezzotti [32]. According to Buchner et al. [31], nanodiamonds as a catalyst can play a crucial role in the growth of moissanite at significantly lower temperatures (~720 °C). The essential step is changing from the supercritical state to the critical/under critical conditions. McMahon et al. [33] give an idea about the growth mechanism of silicon carbide whiskers. The composition and structure of the VLS liquid catalyst (Figure 12) is the task for further sophisticated studies. Noteworthy is that this elliptical catalyst ball is composed of SiC, graphitic carbon, and nanodiamonds. The composition of the liquid phase of the catalyst is unclear - maybe a metastable Bebearing supercritical melt droplet on the growing beryl substrate. Beryl, SiC, and activated nanodiamonds consist, according to Buchner et al. [31], of a diamond core of about 4-5nm, surrounded by a defective carbon shell is noteworthy. The vapor phase, methane, is often seen as a supercritical high-pressure phase inclusion in beryl (see Figure 11). The catalyst ball was mostly partially distorted or destroyed during the further growth of beryl. The occurrence of moissanite whiskers in quartz complicates the genetic interpretation. Another growth mechanism is maybe a low-pressure heteroepitaxial Chemical Vapor Deposition process (CVD). The growth of some large moissanite whiskers starts with a larger gas volume shown in Figure 13.
Figure 12:The supercritical VLS catalyst ball in the starting point for the growth of the SiC whisker in Figure 5a. Carbon (as supercritical methane) and silicon atoms in the supercritical fluid feed the Ge-rich catalyst melt droplet on the beryl substrate.
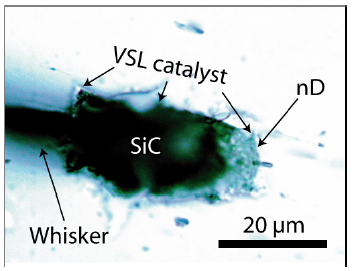
Figure 13:Vapor chamber in beryl (Brl) as the start point of the moissanite (SiC) whisker growth according to a CVD process.
Conclusion
The here-described moissanite, containing nanodiamonds in beryl and quartz, results from the interaction of a berylliumbearing supercritical melt or fluid with the already present Variscan tin mineralization. The supercritical fluid is younger than this tin mineralization [8]. The solubility of Be in a supercritical melt or fluid at temperatures higher than 700 °C is extreme, as [1,2] demonstrated. The extreme physicochemical conditions (at a highly activated state) during the beryl crystallization from a supercritical melt or solution favored the simultaneous crystallization of moissanite with beryl and, to a lesser extent, also in the paragenetic quartz.
The results show different scenarios for the origin of moissanite: ultrahigh-pressure and high-temperature conditions in the mantle region (here, primarily fast-transported mineral spheres) and low-pressure and low-temperature conditions in the upper crust (as SiC whiskers mostly in beryl). Furthermore, our studies show that supercritical fluids or melts are highly complex in composition and change permanently. Many reactions are far away from the equilibrium. All mineral phases we found as spherical inclusions transported by such fluid insert components into such fluid besides water and methane. Further studies are necessary to illuminate these complex processes in more detail. One point is essential: which mechanism is responsible for the extreme enrichment of, for example, beryllium? Are near beryl’s crystallization at supercritical conditions a quantum physical entanglement of Be atoms?
Acknowledgment
We thank the two anonymous reviewers for their constructive comments on an older manuscript version.
References
- Thomas R, Davidson P, Appel K (2019) The enhanced element enrichment in the supercritical states of granite-pegmatite systems. Acta Geochim 38: 335-349.
- Thomas R, Davidson P, Rericha A, Voznyak DK (2022a) Water-rich melt inclusions as "frozen" samples of the supercritical state in granites and pegmatites reveal extreme element enrichment resulting under non-equilibrium conditions. Min J 44: 3-15.
- Thomas R, Davidson P, Rericha A, Recknagel U (2022b) Water-rich coesite in prismatine-granulite from Waldheim/Saxony. Veröffentlichungen Naturkunde Museum Chemnitz 45: 67-80.
- Thomas R, Davidson P, Rericha A, Recknagel U (2023) Ultrahigh-pressure mineral inclusions in a crustal granite: Evidence for a novel transcrustal transport mechanism. Geosciences 13(4): 1-13.
- Proctor JE (2021) The liquid and supercritical fluid states of matter. CRC Press, USA, p. 276.
- Thomas R (2023a) Unusual cassiterite mineralization, related to the Variscan tin-mineralization of the Ehrenfriedersdorf deposit, Germany. Aspects in Mining & Mineral Science 11(2): 1233-1236.
- Keller DS, Ague JJ (2022) Possibilities for misidentification of natural diamond and coesite in metamorphic rocks. Neues Jb- Mineral Abh 197(3): 1276-1293.
- Thomas R (2023b) Growth of SiC whiskers in Beryl by a natural supercritical VLS process. Aspects in Mining & Mineral Science 11(4): 1292-1297.
- Farré-de-Pablo J, Proenza JA, Gonzáles-Jiménez JM, Garcia-Casco A, Colás V, et al. (2018) A shallow origin for diamonds in ophiolitic chromitites. Geology 47(1): 75-78.
- Pujol-Solà N, Garcia-Casco A, Proenza JA, González-Jiménez JM, del Campo A, et al. (2020) Diamond forms during low pressure serpentinization of oceanic lithosphere. Geochemical Perspectives Letters 15: 19-24.
- Yang J, Lian D, Robinson PT, Qui T, Xiong FM, et al. (2020) Geological evidence does not support a shallow origin for diamonds in ophiolite. Acta Geologica Sinica 94: 70-72.
- El Mendili Y, Orberger B, Chateigner D, Bardeau JF, Gascoin S, et al. (2022) Occurrence of SiC and diamond polytypes, chromite and uranophane in breccia from nickel laterites (New Caledonia): Combined Analyses. Minerals 12(196): 1-17.
- Schröcke H (1954) On the paragenesis of tin ore deposits in the Erzgebirge. New J Mineral dep 87(1): 33-109.
- Hosel G (1994) The tin ore deposit area of Ehrenfriedersdorf/Erzgebirge. Mining Monograph, State Office for Environment and Geology. Freiberg, Germany, 1: 195.
- Kumann R, Leeder O (1994) On the relations between granite and ore in the Ehrenfriedersdorf tin deposit, Erzgebirge. Metallogeny of collisional Orogens. Proceedings of the IAGOD Erzgebirge Meeting, Geyer, Germany, In: Seltmann R, Kämpf H, Möller P (Eds.), pp. 166-173.
- O'Bannon III E, Williams Q (2016) Beryl-II, a high-pressure phase of beryl: Raman and luminescence spectroscopy to 16.4 GPa. Phys Chem Minerals 43: 671-687.
- Thomas R, Webster JD, Davidson P (2011) Be-daughter minerals in fluid and melt inclusions: implications for the enrichment of Be in granite-pegmatite systems. Contrib Mineral Petrol 161: 483-495.
- Thomas R, Klemm W (1997) Microthermometric study of silicate melt inclusion in Variscan granites from SE Germany: Volatile contents and entrapment conditions. Journal of Petrology 38(12): 1753-1765.
- Lafuente B, Downs RT, Yang H, Stone N (2016) The power of database. The RRUFF project. In: Armbruster T, Danisi RM (Eds.), Highlights in Mineralogical Crystallography. De Gruyter, Berlin, München, Boston, USA, pp. 1-30.
- Tuschel D (2019) Raman spectroscopy and polymorphism. Spectroscopy 34(3): 10-21.
- Tuschel D (2012) Raman crystallography, in theory and in practice. Spectroscopy 27(3): 2-6.
- Ivanov IG, Yazdanfar M, Lundqvist B, Chen JT, Ul-Hassan J, et al. (2014) High-resolution Raman and luminescence spectroscopy of isotope-pure (SiC)-Si-28-C-12, natural and C-13 – enriched 4H-SIC. Silicon Carbide and Related Materials pp. 471-474.
- Blank VD, Kulnitskiy BA, Perezhogin IA, Tyukalova EV, Denisov VN, et al. (2016) Graphite-to-diamond (13C) direct transition in a diamond anvil high-pressure cell. Int J Nanotechnol 13(8-9): 604-611.
- Al-Tamimi BH, Jabbar II, Al-Tamimi HM (2019) Synthesis and characterization of nanocrystalline diamond from graphite flakes via a cavitation-promoted process. Heliyon 5(5): e01682.
- Roedder E (1984) Fluid inclusions. Reviews in mineralogy. In: Ribbe PH (Ed.), Mineralogical Society of America, 12: 644.
- Petrov DV, Matrosov II, Sedinkin DO, Zaripov AR (2018) Raman spectra of nitrogen, carbon dioxide, and hydrogen in a methane environment. Optics and Spectroscopy 124(1): 8-12.
- Chikvaidze G, Mironova-Ulmane N, Plaude A, Sergeev O (2014) Investigation of silicon carbide polytypes by Raman spectroscopy. Latvian Journal of Physics and Technical Sciences 3: 51-57.
- El Mendili Y, Orberger B, Chateigner D, Bardeau JF, Gascoin S, et al. (2020) Insight into the structural, elastic and electronic properties of a new orthorhombic 6O-SiC polytype. Scientific Reports 10: 7562-7568.
- Schmidt MW, Gao C, Golubkova A, Rohrbach A, Connolly JAD (2014) Natural moissanite (SiC) – a low temperature mineral formed from highly fractionated ultra-reducing COH-fluids. Progress in Earth and Planetary Science 1(27): 1-14.
- Thomas R, Webster JD (2000) Strong tin enrichment in a pegmatite-forming melt. Mineralium Deposita 35: 570-582.
- Buchner F, Kirschbaum T, Venerosy A, Girard H, Arnault JC, et al. (2022) Early dynamics of the emission of solvated electrons from nanodiamonds in water. Nanoscale 14: 17188-17195.
- Frezzotti ML (2019) Diamond growth from organic compounds in hydrous fluids within the Earth. Nature Communications 10: 4962.
- McMahon G, Carpenter GJC, Malis TF (1991) On the growth mechanism of silicon carbide whiskers. Journal of Materials Science 26: 5655-5663.
- Rösler HJ, Baumann L, Jung W (1968) Postmagmatic mineral deposits of the northern edge of the Bohemian Massif (Erzgebirge-Harz). International Geological Congress XXIII Session, Prague. Guide to Excursion 22 AC (c) German Democratic Republic, p. 57.
© 2023 Rainer Thomas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






