- Submissions

Full Text
Aspects in Mining & Mineral Science
Fluoroammonium Method for Processing Scheelite Concentrate
Dyachenko AN*
MIREA - Russian Technological University, Russia
*Corresponding author:Alexander Dyachenko, MIREA - Russian Technological University, Russia
Submission: June 29, 2023: Published: July 07, 2023

ISSN 2578-0255Volume11 Issue5
Opinion
The mineral resource base of tungsten is mainly represented by minerals of the wolframite and scheelite groups, which are of industrial importance [1]. Classical methods of processing scheelite concentrates are based on sintering with soda and subsequent separation of tungsten chemical concentrate on ion-exchange resins [2]. We propose to investigate a new ammonium fluoride method for processing scheelite to reduce the cost of processing and increase the purity of the resulting tungsten product. Synthetic scheelite (CaWO4) was used as a model mixture. The method of sintering scheelite with ammonium bifluoride (NH4F*HF) has been proposed to separate tungsten and calcium. Sintering temperature 200 °C. Ammonium bifluoride was taken with an excess of 10% relative to stoichiometry. The sintering time of scheelite with ammonium bifluoride under laboratory conditions was 2 hours. The process is described by a chemical reaction with the formation of insoluble calcium fluoride.
2CaWO4+ 7NH4F*HF = 2CaF2 + 2(NH4)3WO2F5 + NH3+ 4H2O (1)
Calcium fluoride was separated by filtration, the tungsten-containing solution was studied by Infrared Spectroscopy (IRS) (Figure 1). Next, the tungsten-containing solution was evaporated, and the solid residue was subjected to thermal decomposition.
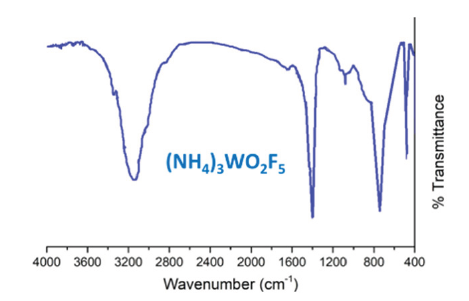
Figure 1:IR spectrum of the obtained sample of the sintering product - wave number 3180cm-1 - NH4+ - wave number 480cm-1 - W-F - wave number 790cm-1 - W-O.
(NH4)3WO2F5 + H2O = WO3+ 5HF + 3NH3 (2)
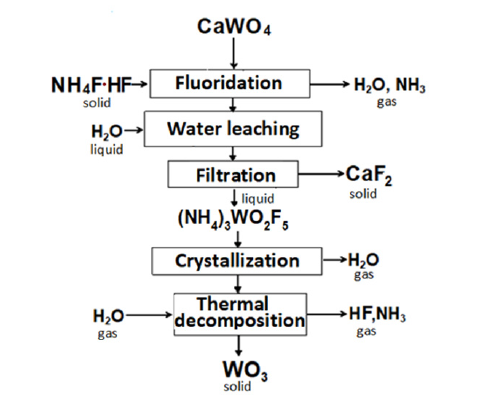
The solid stock was studied by IR spectroscopy (Figure 2). According to the results of the IR spectrum, the substance obtained as a result of the decomposition of (NH4)3WO2F5 is clearly tungsten trioxide (WO3). The IR spectrum also indicates the absence of NH4+, O2- and F- ions. Based on the results of laboratory experiments, a schematic diagram of scheelite processing was proposed [3], (Figure 3).
Figure 2:IR spectrum of the obtained sample of the sintering product.
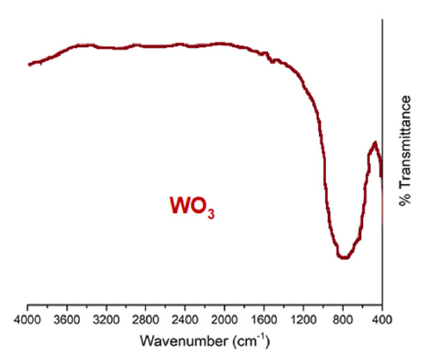
Figure 3:Fluoroammonium scheme for processing scheelite.
Result
a. It was possible to quantitatively decompose scheelite using
ammonium bifluoride and separate solid CaF2 from tungsten
in the form of a (NH4)3WF9 solution.
b. Thermal decomposition of (NH4)3WF9 makes it possible to
obtain pure WO3.
c. The conducted experiments make it possible to start a
laboratory study of natural scheelite concentrates and a
study of the possibility of purifying a tungsten product from
impurities.
References
- (2020) U S Geological Survey, Mineral Commodity Summaries, USA, p. 178.
- Zhao Z, Li J, Wang S, Li H, Liu M, et al. (2011) Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process. Hydrometallurgy 108(1-2): 152-156.
- Laptash NM, Melnichenko EI, Polyshchuk SA, Kaidalova TA (1992) The fluorination of scheelite with ammonium bifluoride. Journal of Thermal Analysis 38(10): 2335-2341.
© 2023 Dyachenko AN. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






