- Submissions

Full Text
Aspects in Mining & Mineral Science
Metal Corrosion Protection in Ammonium Bifluoride-Base Cleaning Agent for Si Contaminants
Teh-Hua Tsai and Chen-Yu Wang*
Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, Taiwan
*Corresponding author:Chen-Yu Wang, Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, Taipei 10608, Taiwan
Submission: May 31, 2023; Published: June 14, 2023

ISSN 2578-0255Volume11 Issue4
Abstract
This research was successful in developing a metal protection mechanism for ammonium bifluoride clean agent that is used in wafer dicing processes, during which metals from IC trace (Al, Cu) and from bumping process (Ti, TiW, Cu, Sn, Ag, etc.) will be exposed. All metals must be protected from the cleaning agent used; any metal corrosion may cause IC to fail or lead to instability, especially when HF molecules are small and can easily dissociate in water. This research showed that the water ratio in the cleaning agent was the main factor that caused metal corrosion. 95% alcohol was then used to replace water in the cleaning agent, and we were able to get a good control of metal corrosion during the cleaning of Si contaminants. These results led to the development of a cleaning agent that could be directly used in the wafer dicing process. In this study, we used bump shear strength as an index of UBM corrosion and got a very quick and correct result.
Keywords:Metal protection; Wafer dicing; Cleaning; Silicon contamination; Ammonium bifluoride
Introduction
In 1970, RCA researchers Kern and Buudinant created the first commercially viable cleaning agent for semiconductors. The semiconductor industry continues to rely on this formulation as the standard wafer cleaning agent. When heated at temperatures between 70 and 80 degrees Celsius, the SC-1 [1,2] formula of NH4OH, H2O2, and H2O in the ratio of 1:1:5 to 1:2:7 may eliminate organic impurities on the wafer. To get rid of any inorganic contamination on the wafer, another SC-2 formula [1,2] calls for a mixture of HCl, H2O2, and H2O in the ratio of 1:1:6 to 1:2:8 at 7080 °C. The oxide layer of the silicon wafer is often removed during the semiconductor IC process using either HF solution or HF vapor as part of a Buffered Oxide Etch (BOE). To get rid of any organic impurities on the wafer, we employed Caro’s acid (a solution of sulfuric acid and hydrogen peroxide) to perform a severe oxidation and dehydration, breaking the carbon-hydrogen link in organic materials. Other methods of cleaning, such as the UV-ozone therapy pioneered by Vig [3], were also developed. Oxygen plasma cleaning is another way for removing contaminants from semiconductor wafers [4]. Those materials and techniques may work well for cleaning semiconductor wafers before dicing, but they don’t appear to be a good fit for cleaning the wafer after dicing.
Wafer dicing using water as a cleaning method leads to silicon contamination. The filthy water is converted into water droplets on the wafer surface when the water start cleaning. The molecules of oxygen in the air will first diffuse to the surface of the wafer and Si impurities, where they will break down into the water droplets. As shown in Equation (1) and Equation (2), silicon atoms on the wafer’s surface and Si impurities will react with oxygen and water molecules (2). Here are the equations of the reactions.
2H2O+Si ⇌ SiO2+4H++4e- (1)
Si+O2 ⇌ SiO2 (2)
As shown in Equations (3) and (4), the produced SiO2 will keep reacting with water to make silicic acid (H2SiO3), and the resulting silicic acid will dissolve uniformly in water.
SiO2+H2O ⇌ H2SiO3 (3)
H2SiO3 ⇌ H++HSiO3- (4)
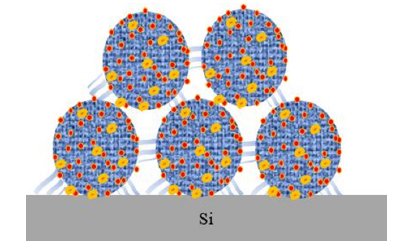
The monomers of silicic acid in the water will slowly polymerize into dimer or trimer complexes as the water evaporates, depositing on the surface of the wafer as insoluble polymers of polysilicic acid [5-10]. The lingering chemicals include polysilicic acid and silicon dioxide [11,12]. Silicon contamination from dicing is similar to water stain, as described in the Figure 1, [6-10]. The SEM image of silicon contaminants shows that there is a concentrated deposit of impurities around the bump, but the area immediately next to the bumps is clean. This observation lends credence to the theory that the reactions in Equations (1) and (2) take place not only on the wafer surface but also on the surface of silicon contamination (Figure 2). In other words, once the water droplets evaporate, the silicon contaminate particles should be uniformly dispersed around the bumps if the reactions did not take place on the surface of the silicon contamination particles. The net structure sketch of silicon contaminants is illustrated in Figure 3, which may explain why cleaning agents of the HF family must be employed for cleaning silicon contamination. The semiconductor industry relies on BOE’s cleaning function since polysilicic acid and silicon dioxide [5] introduce a significant bonding force. To address safety issues, ammonium bifluoride was utilized in place of HF [5]. Even after being employed as a cleaning agent, ammonium bifluoride still has a high corrosiveness toward aluminum and UBM (Ti/TiW) on IC metal surface. When designing a new cleaning solution, protecting metal against corrosion is the most important factor to consider.
Figure 1:The formation of silicon contamination on the wafer [5].
a. The chemical reaction to form silicon contamination on wafer.
b. The state of silicon contamination after water dries off.
c. The microstructure of silicon contamination.
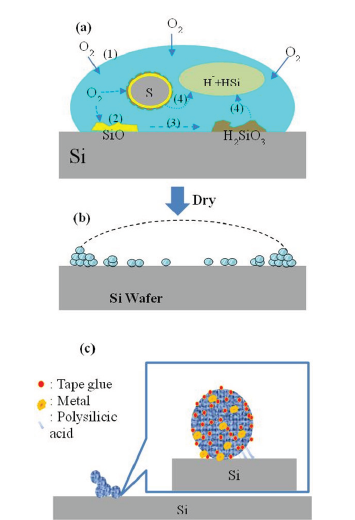
Figure 2:The OM/SEM imaging of silicon contaminants [5].
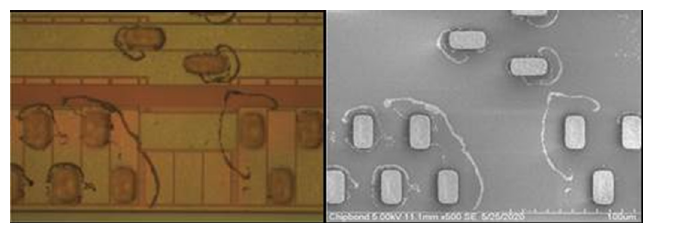
Figure 3:The sketch of silicon contaminants’ NET structure [5].
Material and Methods
Evaluation of corrosion to IC trace (Al)
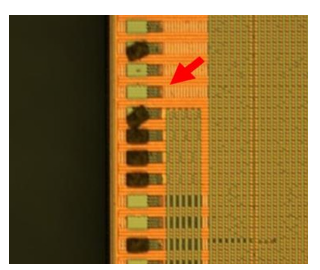
Figure 4:IC trace was damaged at the red arrow spot after cleaning agent dipping test [5].
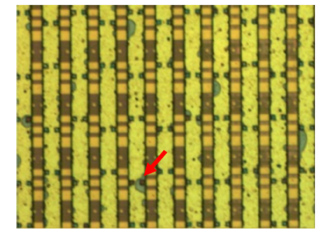
The bumping house receives wafers from the integrated circuit factory. In order to prepare the wafer for metal deposition, the bumping procedure requires a sputter process, which is performed using RF Argon plasma. Cleaning agent should take IC trace protection into account since plasma cleaning [13] can result in passivation layer defects including micro pin holes and micro cracks. In order to make the micro pin hole defect more severe for this study, we reduced the passivation layer thickness by more than 30 percent by repeatedly subjecting the IC samples to RF Argon plasma cleaning. Samples tested with ammonium bifluoride, and phosphoric acid are shown in Figure 4. If the IC trace were damaged like indicated by the red arrow, we knew our sample preparation method was effective.
Figure 5:Bump peel off after 15 minutes of dipping in the cleaning agent of ammonium bifloride mixed with sulfuric acid [5].
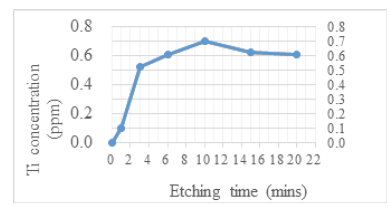
Metals for UBM adhesive layer, Ti and TiW, were utilized in the bumping process to attach aluminum IC open pad. The metal Ti in semiconductors was etched using Hydrofluoric Acid (HF) or an HF-like chemical. The corrosion degree of UBM (Ti/TiW) must be evaluated since ammonium bifluoride is a member of the HF related agents. In Figure 5, we see the outcome of a procedure in which ammonium bifluoride was added to sulfuric acid and then dipped for 15 minutes, after which the defect of peel-off bumps was revealed. It’s tough to secure an actual IC or wafer for metal corrosion testing due to restrictions imposed by the etchant supplier. In most cases, AA (Atomic Spectroscopic Analysis) or ICP (Inductively Coupled Plasma) was used to determine the concentration of metals after real metal was dipped into a cleaning agent solution for different lengths of time (Inductively Coupled Plasma). The resulting curve is seen in Figure 6 below, which shows the results of tests conducted using the finalized cleaning agent formula; these tests revealed that the new cleaning agent provided excellent corrosion protection to the Ti metal, with an etching rate of only around 0.6 ppm after 2 minutes of dipping.
Figure 6:Verification of corrosion rate of Ti metal using the finalized cleaning agent formula in this research, tested by the ICP tool.
Bump shear strength for undercut evaluation
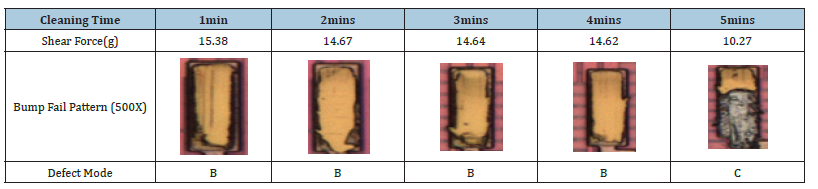
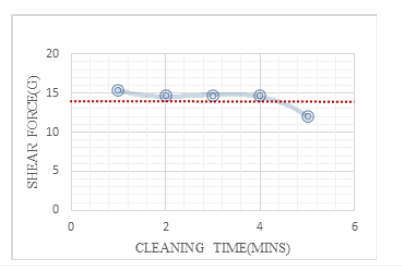
The degree of undercut of a bump can be used as a corrosion index for UBM (Ti/TiW). To get information about the undercut, it would be typical to do a cross section and conduct measurements using a microscope or SEM. Instead of such time-consuming bump undercut approach, we employed a bump shear strength test to quickly determine the corrosion level of various cleaning chemicals. Table 1 shows the results of a shear strength test, in which the increased cleaning time shows that increasing the cleaning time decreases shear strength and alters the shear break mode from B to C. Figure 6 displays the same data as (Table 1) for the bump shear strength curve vs cleaning time. The cleaning agent formula of 1.5% ammonium bifluoride, 10% sulfuric acid, and 38.5% MSA was utilized in this study (Methane Sulfonic Acid). We showed that two indices, shear strength and shear break pattern, may be employed as indices for evaluating novel cleaning agents in the presence of UBM (Ti/TiW) corrosion.
Table 1: Cleaning time vs. bump shear strength and fail pattern.
Result and Discussion
The results indicated that a sufficient test duration and shear
test might help to determine whether or not the new cleaning agent
formula caused corrosion to the UBM.
a. Only thorough cleaning is acceptable.
b. Al corrosion: No corrosion indicates good performance, or else
considered poor performance.
c. UBM undercut: No undercut indicates good performance;
Undercut of less than 1um is acceptable but requires
improvement; Undercut of over 1um is considered poor
performance.
d. Gel residue: No residue indicates good performance, or else
poor performance.
e. Ink marks: Ink mark covers an area of more than 80% indicates
good performance, or else poor performance.
By contrasting the first and second test in Table 2, it was observed that higher concentration of H2SO4 might shield aluminum metal, but not UBM (Ti/TiW) [5,14,15], and that phosphoric acid did not function the same as sulfuric acid. Methane sulfonic acid (MSA) was found to protect UBM metals when compared to Tests 3, 4, and 5 in Table 2, but the solution’s ability to remove contaminants was significantly reduced (possibly due to the inability to dissolve silicon dioxide and polysilicic acid without dissociating ammonium bifluoride) [16]. When Methane Sulfonic Acid was added to a cleaning product, the aluminum’s corrosion-resistant properties were compromised (MSA). We hypothesized that the MSA is a surfactant and that it can lower the surface tension of the passive metal layer, leading to defects or micro-cracks through which acid might seep and directly react with and corrode the pure aluminum metal.
The results of Test 6 in Table 2 showed that substituting 95% ethanol for hazardous methane sulfonic acid in cleaning solution seemed to have no effect on metal protection but was effective for removing UV glue and ink marking (MSA). Substituting 95% ethanol for water in Test 7 of Table 2 yielded excellent results across the board, indicating that the resulting cleaning agent met all specifications and could be utilized in the wafer dicing process without further adjustment. Water leakage and subsequent metal corrosion were reduced in Test 4 compared to Test 7 in Table 2. Another discovery was that polar solvents like water or ethanol were effective at removing silicon contamination, but less polar solvents like MSA were ineffective. Due to the lower polarity of ethanol compared to water, only a negligible quantity of ammonium bifluoride has dissolved in it. The same was true for sulfuric acid in ethanol. This suggested that agents of different polarity can be used as solvent to shield metals from corrosion. Because ammonium bifluoride and sulfuric acid are highly polar agents, their dissociation rate will be reduced in a solvent of lower polarity, potentially leading to a reduced metal corrosion rate but also reduced capacity to remove silicon contamination. MSA has lower polarity that most of the molecules remain in a dissolved state, it follows that MSA is ineffective in dissociating ammonium bifluoride. As a result, the formula of Test 4 in Table 2 was unable to remove the silicon contamination but with no metal corrosion observed. Figure 7 shows a comparison of UBM undercut with and without MSA, revealing that the concentration of H2O, not MSA, as the primary player involved in UBM undercut.
Table 2: Summary of performance for cleaning Si contaminants.
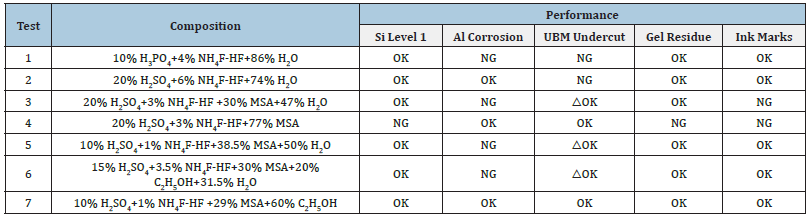
Figure 7:The curve of cleaning time vs. bump shear strength.
Conclusion
Figure 8:Comparison of UBM undercuts by MSA and MSA+Ethanol.
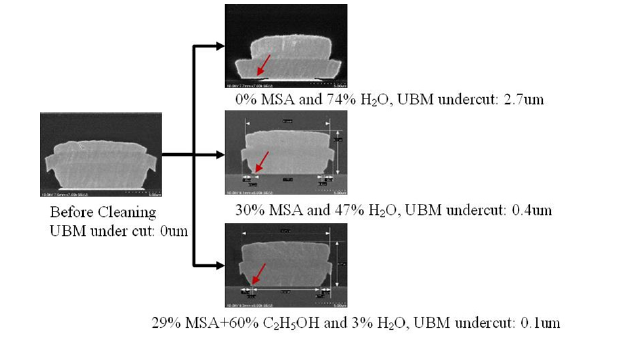
We discovered that the bump shear strength testing method is useful for judging the degree of corrosion on UBM after a prolonged cleaning period (more than 15 minutes). Shear strength or bump break failure mode can help determine the index’s value. Hydrogen ions (H+) are provided by sulfuric acid in the cleaning agent solution. Passivation [14] of metals, including aluminum in this case, is known to occur at a certain concentration of sulfuric acid, which protects the metal against corrosion throughout the cleaning process. Our experiments validated the hypothesis [17,18], indicating that Si contamination may be eliminated using the cleaning agent formulations developed in this research, so long as the cleaning duration is well regulated. When Methane Sulfonic Acid is added to a cleaning product, the aluminum’s corrosionresistant properties may be compromised (MSA). We hypothesized that since MSA is a surfactant and can lower the surface tension of the passive metal layer, it will lead to defects or micro-cracks through which acid can seep and directly react with and corrode the pure aluminum metal Figure 8.
The corrosion of the chip’s circuit metal (Al) and under-bump metal (Ti/TiW) may be prevented if MSA or ethanol were used instead of water. Since ammonium bifluoride is protected from corrosion and becomes more difficult to dissolve in MSA or ethanol [19], this may be the reason why it is less effective in removing silicon impurities. Since ethanol may substitute for water, it will not reduce the cleaning strength when dealing with Si contamination, as the metal is still shielded from corrosion [19,20]. This research demonstrated that the under-bump metal (Ti/TiW) and chip’s circuit metal (Al) were able to resist corrosion by the cleaning agent when MSA was mixed with ethanol, and this effect persisted even when the cleaning duration was over-regulated. In addition to protecting metal from corrosion, the new cleaning agent mix also made it simple to eliminate Si contamination, UV glue, and ink mark. These findings provided credence to the viability of the proposed cleaning agent formula for removing silicon contamination after dicing.
Acknowledgement
The analysis tools support from CB analysis lab and members are greatly acknowledged.
References
- Kern W, Puotinen DA (1970) Cleaning solutions based on hydrogen peroxide for use in silicon semiconductor technology. RCA Rev 31: 187-206.
- Ruzyllo J, Novak RE (1994) Cleaning technology in semiconductor device manufacturing. The Electrochemical Society, Incorporated, Proceedings 94-97: P3-P10.
- Vig JB (1985) UV ozone cleaning of surfaces. J Vac Sci Technol A 3: 1027-1034.
- Oehrlein GS, Scilla GJ, Jeng SJ (1988) Efficiency of oxygen plasma cleaning of reactive ion damaged silicon surfaces. Appl Phys Lett 52: 907-909.
- Tsai TH, Wang CY (2023) A study of ammonium bifluoride as an agent for cleaning silicon contamination in the wafer dicing process. Appl Sci 13(9): 5294.
- Liu X, Liu C, Meng C (2019) Oligomerization of silicic acids in neutral aqueous solution: A first-principles investigation. Int J Mol Sci 20(12): 3037.
- Putz MV, Russo N, Sicilia E (2004) On the applicability of the HSAB principle through the use of improved computational schemes for chemical hardness evaluation. J Comput Chem 25(7): 994-1003.
- Putz MV (2008) Density functionals of chemical bonding. Int J Mol Sci 9(6): 1050-1095.
- Putz MV (2008) Maximum hardness index of quantum acid-base bonding. MATCH Commun Math Comput Chem 60: 845-868.
- Putz MV (2011) Chemical action concept and principle. MATCH Commun Math Comput Chem 66: 35-63.
- Mondal B, Ghosh D, Das AK (2009) Thermochemistry for silicic acid formation reaction: Prediction of new reaction pathway. Chem Phys Lett 478(4-6): 115-119.
- Goto K (1956) Effect of pH on polymerization of silicic acid. J Phys Chem 60(7): 1007-1008.
- Ku CM, Cheng S (2022) Factor design for the oxide etching process to reduce edge particle contamination in capacitively coupled plasma etching equipment. Appl Sci 12(11): 5684.
- Lin CC, Wuu DS, Huang JJ (2019) A study of aluminum oxide passivation films by liquid phase deposition and its application for ultraviolet solid-liquid heterojunction photo detectors. National Chung Hsing University of Materials Science and Engineering, Taiwan.
- Affrossman S, Daviot J, Holmes D, Pethrick RA, Wilson M (2001) Molecular design for inhibition of titanium corrosion in resist cleaner systems. Corros Sci 43(5): 939-950.
- Hossain ST, Johra FT, Jung WG (2018) Fabrication of silicon carbide from recycled silicon wafer cutting sludge and its purification. Appl Sci 8: 1841.
- Huang JJ, Lin CH, Ho YR, Chang YH (2019) A study of aluminum oxide passivation films by liquid phase deposition and its application for ultraviolet solid-liquid hetero junction photodetectors. Surf Coat Technol 391: 125684.
- Mirhashemihaghighi S, Światowska J, Maurice V, Seyeux A, Zanna S, et al. (2016) Corrosion protection of aluminum by ultra-thin atomic layer deposited alumina coatings. Corros Sci 106: 16-24.
- Shufeng Y, Peisheng Y, Jinjian D (2022) Dry-type fluorination device and terbium fluoride preparation method. China Patent, No: CN-114100529-A.
- Reda R (2017) Corrosion & protection of metals. Ph D in Metallurgical and Materials Science Engineering, 27: P9-P15.
© 2023 Chen-Yu Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






