- Submissions

Full Text
Aspects in Mining & Mineral Science
Unusual Cassiterite Mineralization, Related to the Variscan Tin-Mineralization of the Ehrenfriedersdorf Deposit, Germany
Rainer Thomas*
Im Waldwinkel 8 D-14662 Friesack, Germany
*Corresponding author:Rainer Thomas, Im Waldwinkel 8 D-14662 Friesack, Germany
Submission: April 18, 2023; Published: April 28, 2023

ISSN 2578-0255Volume11 Issue2
Abstract
This paper shows an unusual occurrence of orthorhombic cassiterite in a classic Variscan tin mineralization of the Erzgebirge, where the cassiterite typically crystallizes in the tetragonal system. The characteristic Raman spectra of orthorhombic cassiterite are shown for the first time. Furthermore, the origin of this cassiterite as a result of the interaction of a tin deposit with supercritical melts or fluids is discussed.
Keywords:Tetragonal and orthorhombic cassiterite; Raman spectroscopy; Supercritical melts/fluids; OH-rich topaz
Introduction
In Romer et al. [1] found for the granite-related tin mineralization in the Erzgebirge using U-Pb, Th-U-Pb, Rb-Sr, and Re-Os dating for the Sauberg mine near Ehrenfriedersdorf an age of about 320.6±1,9 and 319.7±3.4Ma. This data does not support an extensive age range for the Late-Variscan granites and directly related tin-tungsten ore deposits. In the same paper, the authors gave for uraninite, monazite, and xenotime from the Ehrenfriedersdorf pegmatite (Qu8) identical chemical ages: 320±4.0Ma (n=43 crystals) – see also Rhede et al. [2] At the same time, the present author found with Dieter Rhede (unpublished data) a sample from the tin mineralization of the Sauberg mine (Ehrenfriedersdorf), which does not fit this general age statement. The chemical U/Pb and Th/Pb age determination on xenotime inclusions in cassiterite give a mean of 137±21Ma (n=50). The simplest explanation is Pb diffusion loss by significant heating. However, we had no idea of such a mechanism at that time. Therefore, the data were not considered.
The newest work on the Greifenstein granite [3] opened a way to solve this dilemma. Very hot supercritical fluids or melts coming from mantle depths bring with their load also the necessary energy for the disturbance of the isotope equilibrium. Because there is not clear what happens at such an energy influx, we would not call this number a defined age. More work is necessary. Here it will be shown only the proofs and the results of such interaction.
Sample material
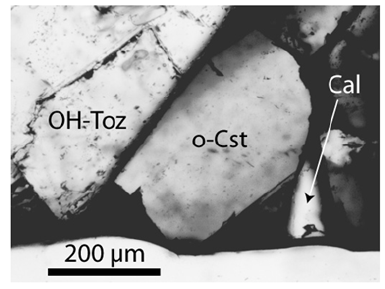
The study used a large cassiterite crystal of about 40x20mm (Sample Sn-70 from the Prinzler countervein (Puffe, 1936)). The root zone contains tiny topaz crystals (about 2mm in diameter). Besides cassiterite and topaz, there are a lot of other minor minerals: plagioclase, albite, kumdykolite, calcite, fluorite, graphite, rynersonite, mangancolumbite, uraninite, monazite, xenotime, moissanite, diamond, Ti-carbides. The last three minerals are generally associated with small black elliptical aggregates in topaz. The sample contains in muscovite tiny cassiterite crystals with a clear rhombic cross-section (Figure 1). Larger crystals have no precise crystallographic forms (Figure 2).
Figure 1:An orthorhombic cassiterite crystal in muscovite (sample Sn-70).
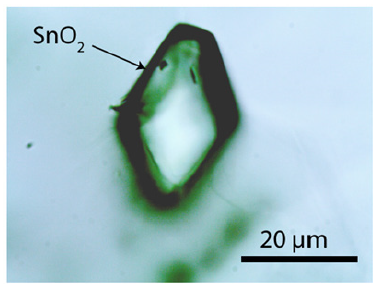
Figure 2:Orthorhombic cassiterite (o-Cst) in the root zone of a larger cassiterite crystal. OH-Toz is an OHrich topaz, and Cal is calcite.
Microscopy and Raman spectroscopy: methodology
The composition of F-rich topaz, uraninite, monazite, and xenotime was determined with microprobe techniques (see [1]). A detailed description and discussion of these results will not be given here. All microscopic studies were performed with a petrographic polarization microscope and the Raman spectrometer. The Raman spectra were recorded with an EnSpectr Raman microscope RamMics M532 in the spectral range of 0–4000cm−1 using a 50mW single-mode 532nm laser, entrance aperture of 20μm, holographic grating of 1800g/mm, and spectral resolution ranging from 4–6cm−1. Depending on the grain size, microscope objective lenses with a magnification varying from 3.2x to 100x were used. As a 100x objective lens, the Olympus long-distance LMPLFLN100x was applied. The laser energy on the sample can be adjusted down to 0.02mW. The Raman band positions were calibrated before and after each series of measurements using the Si band of a semiconductorgrade silicon single-crystal. The run-to-run repeatability of the line position (based on 20 measurements each) was ±0.3cm−1 for Si (520.4±0.3cm−1) and 0.5cm−1 for diamond (1332.3±0.5cm−1 over the range of 80–2000cm−1). A water-free natural diamond crystal as a diamond reference was used (for more information, see [4]).
Results
Topaz
At the beginning of the study, it was conspicuous that the topaz of the studied cassiterite sample was very OH-rich. In contrast, the topaz of the tin-tungsten mineralization in the Erzgebirge is generally F-rich. From twenty microprobe analyses on F-rich topaz from the Sauberg deposit, the following mean formula for topaz was obtained:
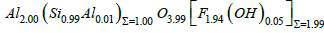
Figure 3:Sub-spherical inclusion in OH-rich topaz composed from carbonic material (graphite), moissanite,and diamond
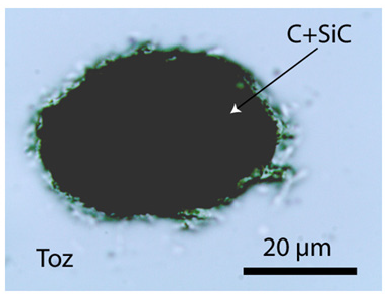
Figure 4:Spherical kumdykolite inclusion with carbonic material in OH-rich topaz (Toz).
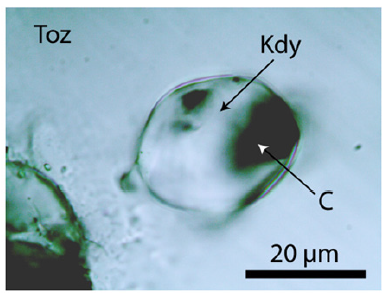
The OH of the topaz in question was estimated from the relationship between the ratio of the integral intensity of the Raman lines ~933cm-1 and ~3650cm-1 and the OH concentration. As references, the yellow water clear topaz from Ouro Preto (Brazil) and the pink topaz from Katlang district (Pakistan) was used – see Thomas [5] and Pinheiro et al. [6]. As a first estimation, a value for XOH=60±10 % result (n=11 different crystals) is remarkably high. However, a second topaz, maybe OH saturated, corresponds to Al2SiO4(OH)2. Such OH-rich topazes occur, according to Xue et al. [7] only at high temperatures and pressures (~1000 °C and 10GPa). The high-pressure origin of the OH-rich topaz is indicated by the occurrence of many spherical aggregates of graphite, moissanite, and diamond (Figure 3), as well as the high-temperature feldspar kumdykolite [NaAlSi3O8] (Figure 4).
Cassiterite
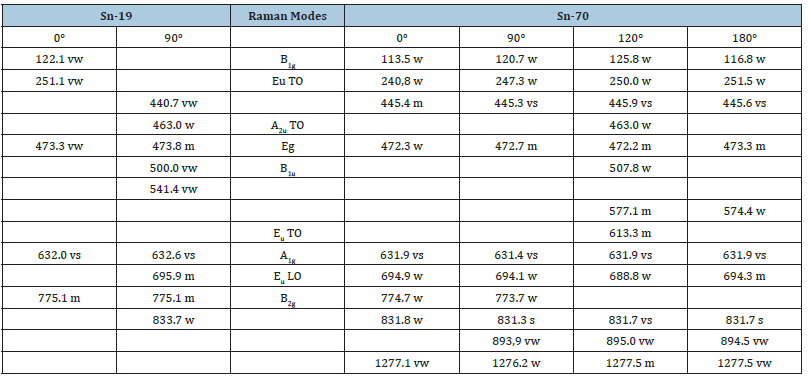
The study of cassiterite in this sample brought some surprising results: The first point is the abundance of carbonic material as inclusions, often as small spherical or elliptical aggregates, containing microcrystals of diamond and moissanite, different from the technical SiC used for preparation (Raman bands at 145.4s, 235.0m, 260.6m, 773.7vs, 954.2scm-1, respectively). However, more unexpected was the Raman spectrum of the cassiterite crystals. Cassiterite generally crystallizes in the tetragonal crystal system. The Raman spectrum of tetragonal cassiterite is characterized (see [8,9]) by a strong band at about 634cm-1 (A1g mode) and relatively weak bands between 123 and 782cm-1. Table 1 & Figure 5 shows the frequencies of different Raman modes of the two cassiterite types, Sn-19 and Sn-70. The sample Sn-19 is a typical tetragonal cassiterite crystal from Ehrenfriedersdorf (see [5]). About 50 cassiterite crystals from different deposits worldwide were tested, and all show a similar Raman spectrum with the dominant 632cm-1 band. Only the Raman spectrum of the cassiterite sample Sn-70 is characterized by three strong to very strong bands at 446, 632, and 832cm-1.
Figure 5:Typical Raman spectra of tetragonal (a) and orthorhombic (b) cassiterite.
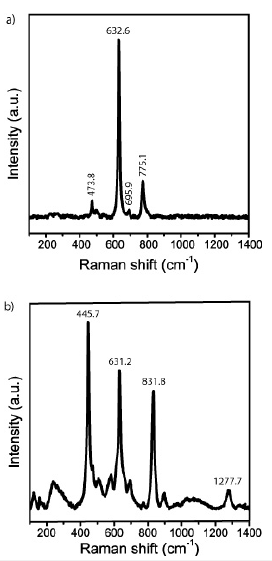
Table 1:Frequencies of different Raman modes of large tetragonal cassiterites (Sn-19) and frequencies of orthorhombic cassiterite (Sn-70), measured at different azimuth positions. Intensity: vs–very strong, s–strong, w–weak, m–medium, w–weak, vw–very weak. TO–Transverse Optical, LO–Longitudinal Optical.
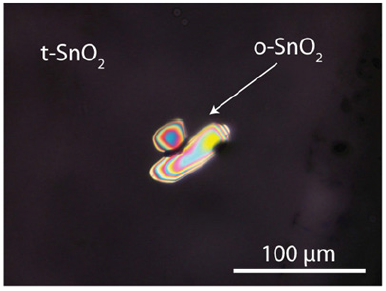
However, after careful screening, many cassiterite crystals contain tiny ones with orthorhombic cross-sections (Figure 6). These crystals show a very strong birefringence and the typical and strong Raman bands at 446, 632, and 832cm-1. Therefore, these crystals can be interpreted as remnants of earlier history. Inclusions of orthorhombic cassiterite crystals in tetragonal cassiterite are widespread in Ehrenfriedersdorf and Schlaggenwald (Horni Slavkov, Chech Republic).
Figure 6:Inclusions of orthorhombic (o-SnO2) in tetragonal (t-SnO2) cassiterite (crossed Nicols).
Discussion
In Lye [10] described rhombic cassiterite from the Phuket region (Malaysia). This cassiterite is of pegmatitic origin, formed at high temperatures (500 to 600 °C) and high pressures. Unfortunately, the author represented no Raman spectra from this material. Essential for our discussion of the origin of the Sn-70 sample is the high-pressure hints. The topaz is high in OH (XOH=60±10 %), and such topazes are not typical for hydrothermal conditions. Maybe high pressures are necessary to form such OH-rich topaz [11]. Further hints for the effect of high pressure come from the proof of fast-rising supercritical fluids or melting from mantle depths [3]. Diamond, graphite, and moissanite inclusions in topaz indicate them. There are two possibilities. First, the supercritical fluids also bring cassiterite from greater depths, which must be the highpressure form: the orthorhombic SnO2-II. According to Suito et al., 1975 at 800 °C (minimum temperature for the supercritical fluid), the equilibrium pressure is 15.8GPa. Second, the supercritical fluid initiates a re-distribution of the already present cassiterite. The relatively high concentration of carbonic material in the supercritical fluid transported tin as an Sn2+ complex. That is consistent with our results on the extreme element enrichment via supercritical melts or fluids [12].
References
- Romer RL, Thomas R, Stein HJ, Rhede D (2007) Dating multiply overprinted Sn-mineralized granites – examples from the Erzgebirge, Germany. Miner Deposita 42: 337-359.
- Rhede D, Wendt I, Förster HJ (1996) A three-dimensional method for calculating independent chemical U/Pb- and Th/Pb-ages of accessory minerals. Chemical Geology 130(3/4): 247-253.
- Thomas R, Davidson P, Rericha A, Recknagel U (2023) Ultrahigh-pressure mineral inclusions in a crustal granite: Evidence for a novel transcrustal transport mechanism, Geosciences 13(4): 1-13.
- Thomas R, Davidson P, Rericha A, Recknagel U (2022a) Water-rich coesite in prismatine-granulite from Waldheim/Saxony. Veröffentlichungen Nat Mus Chemnitz 45: 67-89.
- Thomas R (1982) Results of the thermos barometric investigations on liquid inclusions in minerals of the post-magmatic tin-tungsten mineralization of the Ore Mountains. FFH C 370: 85.
- Pinheiro MVB, Fantini C, Krambrock K, Persiano AIC, Dantas MSS, et al. (2002) OH/F substitution in topaz studied by Raman spectroscopy. Physical Review B 65(10): 104301-104306.
- Xue X, Kanzaki M, Fujui H (2010) Unique crystal chemistry of two polymorphs of topaz-OH: A multi-nuclear NMR and Raman study. Am Miner 95: 1276-1293.
- Diéguez A, Romano-Rodriguez A, Vilà A, Morante JR (2001) The complete Raman spectrum of nanometric SnO2 Journal of Applied Physics 90(3): 1550-1557.
- Wang R, Wu J, Dubessy J (1993) Raman spectroscopy of Nb, Ta-rich cassiterite in Beauvoir and Montebras granites, France. Chinese Journal of Geochemistry 12(4): 353-360.
- Lye YH (1984) Studies of pegmatitic cassiterites from the Gunung Jerai (Kedah), Bakri (Johore) and Kathu Valley (Phuket) regions. Geol Soc Malaysia, Bulletin 17: 107-161.
- Wunder B, Andrut M, Wirth R (1999) High-pressure synthesis and properties of OH-rich topaz. Eur J Mineral 11(5): 803-813.
- Thomas R, Davidson P, Rericha A, Voznyak DK (2022b) Water-rich melt inclusions as “frozen” samples of the supercritical state in granites and pegmatites reveal extreme element enrichment resulting under non-equilibrium conditions. Min J (Ukraine) 44: 3-15.
© 2023 Rainer Thomas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






