- Submissions

Full Text
Aspects in Mining & Mineral Science
Nano-Nickel as a Catalyst for the Ni/ PS/Si Hydrogen Fuel cells
Dzhafarov TD*, Bayramov AH, Ragimov SX, Bagiyev EA, Asadullayeva SG, Jalilli JN and Amiraslanov IR
Institute of Physics, Ministry of Science and Education of Republic of Azerbaijan, Baku, Azerbaijan
*Corresponding author:Dzhafarov TD, Institute of Physics, Ministry of Science and Education of Republic of Azerbaijan, Baku, Azerbaijan
Submission: March 23, 2023; Published: April 11, 2023

ISSN 2578-0255Volume11 Issue2
Abstract
In this paper we report the results on fabrication and output characteristic of novel Direct Ammonia Fuel Cells (DAFC) based on Porous Silicon (PS)/nano-nickel catalyst structure. Output parameters of the structures were measured at Ammonia Solution (AS) concentration of 0-25%. AS/Ni/PS/Si fuel cell exhibits the open-circuit voltage of 0.85V and peak power density of 0.66mW/cm2 at ammonia solution concentration of 25% at room temperature. Inverse dependence of output power density on Ni film thickness was observed. Mechanisms of generation of the electricity, as well as Ni thickness dependence in Ni/PS/Si cells, exposed directly hydrogen-containing composition as a fuel are discussed.
Keywords:Nano-Ni/porous silicon/Si fuel cell; Ammonia electrolyte; Power density; Open-circuit voltage
Introduction
Hydrogen is an important chemical material that is utilized on a large scale in synthetic chemical industries in modern society. On the other hand, technologies for utilizing hydrogen as clean sources of energy are considered to assume an important position in order to overcome problems of lack of energy and environment in future. Energy obtained upon a reaction of hydrogen and oxygen is directly converted into electric energy. Fuel cells offer a significant advantage over traditional combusting-thermal energy conversion, so they provide efficiencies of electrical power supply in the range of 35 to 55%, causing very low level of pollutant emission. They can be used in a wide variety of applications from miniaturized portable power to stationary power stations [1,2]. Nowadays the rise in portable electronics requires energy sources compatible with environmental constraints. Proton Exchange Membrane (PEM)-type fuel cell is attractive and alternative option for production of clean energy for portable and transportation applications [3]. It consists of an organic polymer membrane as proton conductor (Nafion) sandwiched between two platinum-based catalysts. Fuel cells operate under clear hydrogen exposition at 100-300 °C. An external reformer is required to convert fuel such as methanol or natural gas to hydrogen. However, the major hurdle for commercialization of system with PEM fuel cell consists of generation and direct supply of pure hydrogen gas. Recently, Direct Methanol Fuel Cell (DMFC), based on the PEMtype cell appropriate for micro-scale electronics power has been developed [4-6]. Using the organic polymer membrane in PEM-type direct-fueled cells and the high-cost platinum catalysts mitigates durability and increases the cost of fuel cells. Degradation of structural characteristics of polymer membrane material due to diffusion and electro-corrosion processes during electrochemical energy conversion in fuel cells becomes the major challenge in fuel cell basic research. Now, the fabrication technology of most portable electronic devices is based on standard micro-fabrication technique, but PEM-type fuel cells with polymer membrane are not readily integrated with this technique. On this view, it would be desirable to develop an inorganic proton conducting membrane for fuel cell with technology compatible with standard micro-fabrication techniques. Schottky-type fuel cell for micro power generation, based on nano (micro)-porous silicon, operates at room temperature under direct exposition of hydrogen-containing gas and liquid compounds [7,8]. Fabrication of porous silicon is compatible with micro-fabrication technology. Moreover, high porosity of porous silicon and very large surfaceto- volume ratio (103m2.cm-3) ensures the high proton conductivity comparable with that of polymer membrane Nafion. Additionally, such direct-fueled fuel cells can operate at room temperature under exposition hydrogen-containing compounds (including the sea-water) without formation of polluting gases. Low-temperature fuel cells with proton-exchanged membranes consists of a protontransmitting membrane and platinum or palinode catalyst which separate two electrodes-cathode and anode. The molecular hydrogen is dissociated on the catalyst anode, the protons transmit through the membrane to cathode, but electrons go to external circuit. Dissociation rate of the molecular hydrogen on the anode depends on catalyst efficiency (electron work function of the element). The highest value of the work function has platinum (5.33-5.55eV) therefore the most amount of electrons release on the platinum anode. It should be noted that in the commercial fuel cells with nominal output voltage about 900V as a catalyst is used the platinum which is too expensive for this purpose. Therefore in recent years considerable attention has been focused on noble metal free catalysts which are much cheaper than platinum one [9]. In this paper the fabrication details and room temperature characteristics of new type direct ammonia fuel cells performed using proton transmitting porous silicon membrane and cheap nano-nickel catalyst are reported.
Experimental Procedures
Porous silicon layers with thickness of 10-40μm and average porosity from 40% to 70% were prepared on n-type monocrystalline (111) Si substrates with resistivity of 170Ω∙cm by anodic etching in hydrofluoric-ethanol solution under the white light illumination [8]. The Ni/PS/Si structures were fabricated by evaporation of thin Ni films onto the PS/Si structure at room temperature by using Mo bout in vacuum of 1.3×10−3Pa. The thickness of deposited nickel films (10-100nm) was controlled during the deposition process by Inficon. Two type fuel cells structures-fuel cells with delicate thickness of nickel (Ni(20nm)/PS/Si),“nano-nickel” and fuel cells with normal thickness of nickel (Ni(100nm)/PS/Si/, “normal nickel”) were studied. Electrical measurements of fuel cells were carried out using ammonia solution (NH3:H2O) of different concentrations (0-25%). The composition of the ammonium solution changed with the addition of water. Ammonia and water form ammonium hydroxide on the surface of nickel:
NH3+H2O=NH4OH (1)
The crystalline structure of layers was studied by X-Ray Diffraction (XRD) measurements (Bruker XRD D2 PHASER (Germany) Diffractometer in 2θ of 10-70°). The current-voltage characteristics, open-circuit voltage (Voc) and short-circuit current density (J) of the Ni/PS/Si cells were measured at room ambient (40% RH) as well as in ammonia solution. The generated by ammonia open-circuit voltage and short-circuit current between the contacts on Ni and Si substrate were measured directly by digital multi-meter (Thurlby-1503). It should be noted that the structures did not exhibit any noticeable sensitivity under illumination of 120mW/cm2 therefore ammonia-stimulated measurements of current-voltage characteristics were performed under daylight conditions. All of the measurements were performed at room temperatures.
Results and Discussion
Figure 1:XRD pattern of Ni film of 20nm thickness on glass substrate.
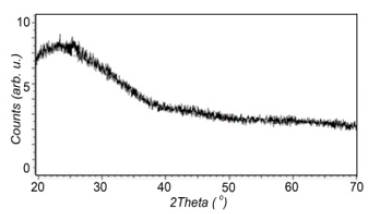
Figure 2:Current density-voltage characteristics (1) and power density (2) of AS/Ni(20nm)/PS/Si cells (nano-nickel) with 25% ammonia solution as fuel.
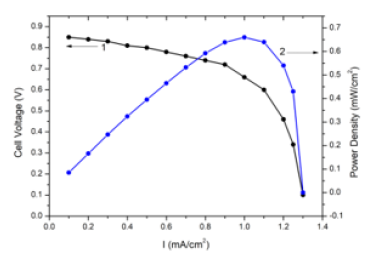
Figure 1 shows the XRD pattern of Ni film of 20nm thickness on glass substrate. No reflections were observed in the Ni sample. Consequently, the Ni layers in the sample have a disordered (amorphous) structure. Obviously, such a structure is associated with very small grain sizes (a few nm) because of low thickness and small ionic radius of Ni+2 (0.083nm). Figures 2 & 3 show current density-voltage characteristics of two structures, AS/Ni(20nm)/ PS/Si and AS/Ni(100nm)/PS/Si cells with 25% ammonia solution as fuel. From (Figure 2) it is seen that AS/Ni(20nm)/PS/Si cell (with nanonickel) under ammonia solution produce electricity (the opencircuit voltage U=850V, the short-circuit current density J=1.34mA/ cm2, and maximum power density 0.66 mW/cm2). Whereas AS/Ni (100nm)/PS/Si cell (with normal nickel) under ammonia solution produce electricity (the open-circuit voltage U=640V, the shortcircuit current density J=0.85 mA/cm2, and maximum power density 0.26mW/cm2) (Figure 3). From the comparison of two results, it is seen that the fuel cell with nanonickel catalysts (20nm) has much higher output power density (very close to the cells with platinum catalyst) than that cell with normal thickness (100nm) catalyst. The open-circuit voltage (1) and short-circuit current density (2) of AS/Ni(20nm)/PS/Si cell (with nanonickel) for different ammonia concentrations are presented in Figure 4. It can be seen that an increase in the ammonia concentration in the range of 0-25% is accompanied by a nonlinear increase in both the circuit voltage and the short-circuit current density. Thus, the following experimental facts were obtained as a result of study of Ni/PS/Si-based fuel cells with ammonium hydroxide solution (ammonia solution) at room temperature:
Figure 3:Current density-voltage characteristics (1) and power density (2) of AS/Ni(100nm)/PS/Si cells (normal nickel) with 25% ammonia solution as fuel.
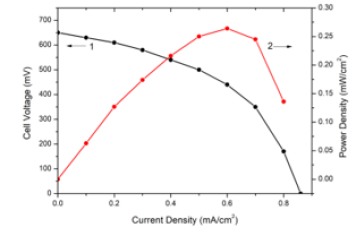
Figure 4:Open-circuit voltage (1) and short-circuit current density (2) of AS/Ni(20nm)/PS/Si cell (nano nickel) vs ammonia concentration.
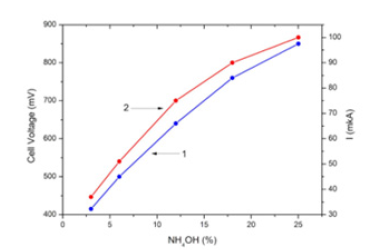
a. Formation of electricity was observed for Ni/PS/Si cells placed in ammonia solution. b. Output power density of Ni/PS/Si cells placed in ammonia solution strongly depends on the thickness of Ni catalyst. AS/Ni(20nm)/PS/Si cell (with nanonickel) under ammonia solution produce electricity with maximum power density 0.66mW/cm2, whereas AS/Ni(100nm)/PS/Si cell (with normal nickel) with maximum power density 0.26mW/cm2. c. The open circuit voltage and short-circuit current density of Ni/PS/Si cells increase with increasing of the ammonia concentration in the range of 0-25%.
The mechanism of electricity generation in the investigated cells under the action of an ammonia solution is similar to the previously proposed generation mechanism for the Ag/PS/Si element directly exposed to hydrogen or a hydrogen-containing composition as a fuel [10]. In this case electrochemical reactions proceeding in direct ammonia fuel cell with proton-conducting porous silicon can be expressed as
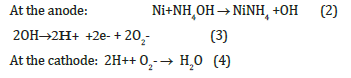
The electrons and protons formed in Ni catalyst films as a result of splitting of hydrogen pass through the external circuit and porous silicon membrane layer, respectively, and reach the cathode (PS/ Si interface). The higher output parameters in case of nano-nickel catalyst can be explained by close proximity of protons, formed after dissociation of hydrogen, to the membrane, that makes easier the transmission of protons trough the membrane.
Conclusion
AS/Ni(20nm)/PS/Si cell (with nanonickel) under ammonia solution produce electricity (the open-circuit voltage U=850V, the short-circuit current density J=1.34mA/cm2, and maximum power density 0.66mW/cm2), whereas AS/Ni(100nm)/PS/Si cell (with normal nickel) under ammonia solution produce electricity (the open-circuit voltage U=640V, the short-circuit current density J=0.85mA/cm2, and maximum power density 0.26mW/cm2) at room temperature. The higher output parameters of cells in case of nanonickel catalyst can be explained by close proximity of protons, formed after dissociation of hydrogen, to the membrane, that makes easier the transmission of protons through the membrane. These results demonstrate the feasibility of development of low-cost (as compared with platinum based cells) Ni/PS-based micro fuel cell for portable electronic application.
References
- Vielstich W, Lamm A, Costeiger NA (Eds.), (2003) Handbook of fuel cell: Fundamentals, technology, applications. 1(4): New Jersey, US.
- Vielstich W, Costeiger NA, Yokoko H (Eds.), (2009) Handbook of fuel cell: Advances in electroc-catalists, materials, diagnostics and Durability. 5(6): Wiley, Hoboken, US.
- Hokaday RC (2000) Microfuel cell for portable electronics. 35(44): Manhattah Scienifics, Power, Santiago, Chile.
- Varga A (2007) Chapter 1 introduction to fuel cell technology, In: Kuang K, Easter K (Eds.), Fuel cell electronics packaging. Springer, Berlin, Germany, 145(163).
- Ohashi M, Guo Z, Wang N, Tseng KN (2010) Small DMFG for portable applications. Fujika Technical Review (1): 44-46.
- Kamarudin SK, Akhmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DFMC) for portable electronic devices. International Journal of Hydregen Energy 34(16): 6902-6916.
- Dzhafarov TD, Bayramov AH (2017) Porous silicon and solar cells. In: Canham L (Ed.), Springer, Germany, p. 1479.
- Dzhafarov TD, Aydin YS, Oruc LC (2008) Porous silicon-based gas sensors and miniature hydrogen cells. Jpn J Appl Phys 47(10): 8204-8207.
- Oshchepkov AG, Braesch G, Amara SO, Rostamikia G, Maranzana G, et al. (2019) Nickel metal nanoparticles as anode electrocatalysts for highly efficient direct borohydride fuel cells. ACS Catal 9: 8520-8528.
- Dzhafarov TD, Bayramov AH, Sadigov MS, Ragimov SX, Bagiyev EA, et al. (2022) Ag/porous silicon-based ammonia-fed fuel cells. Aspects in Mining and Mineral Science 10(3): 1152-1155.
© 2023 Dzhafarov TD. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






