- Submissions

Full Text
Aspects in Mining & Mineral Science
Ag/Porous Silicon-Based Ammonia-Fed Fuel Cells
Dzhafarov TD*, Bayramov AH, Sadigov MS, Ragimov SX, Bagiyev EA, Asadullayeva SG, Jalilli JN and Amiraslanov IR
Institute of Physics, Ministry of Science and Education Republic of Azerbaijan, Azerbaijan
*Corresponding author: Dzhafarov TD, Institute of Physics, Ministry of Science and Education Republic of Azerbaijan, Baku, Azerbaijan
Submission: November 10, 2022;Published: November 29, 2022

ISSN 2578-0255Volume10 Issue3
Abstract
In this paper the results on fabrication technology, structural, luminescent and electrical characteristics of Ag/PS/Si structures as direct ammonia fuel cells are reported. Here nano- Porous Silicon (PS) acts as proton conducting membrane, Ag as catalyst layer. X-Ray Diffraction (XRD), electrical, Photoluminescence (PL) measurements and performance characteristics of Ag/PS/Si cells were studied. Generation of electricity was observed for Ag/PS/Si cells placed in ammonia solution in the range 0-25% at room temperature. Ag/PS/Si cells exhibited the open-circuit voltage of 0.59V and peak power density of 47.2μW/cm2 with ammonia solution of 25% as fuel at room temperature. Mechanisms of generation of the electricity in Ag/PS/Si cells, exposed directly hydrogen-containing composition as fuel are considered. The advantage of investigated direct ammonia fuel cells consists in simplicity of fabrication technology, which can be integrated into standard silicon micro fabrication and operation of cells at room temperature.
Keywords:Ag/porous silicon/Si fuel cell; Ammonia electrolyte; Power density; Open-circuit voltage
Abbreviations: XRD: X-Ray Diffraction; PEM: Polymer Electrolyte Membrane; PS: Porous Silicon; PL: Photoluminescence
Introduction
A fuel cell is an electrochemical device (a galvanic cell) which converts the energy of a chemical reaction into electrical energy. It usually consists of two electrodes, a negative electrode (or anode) and a positive electrode (or cathode) sandwiched around an electrolyte. In dependence of electrolyte, different types of hydrogen fuel cells have been intensively investigated as the effective sources of clean electric energy. This classification determines the electrochemical reactions, the type of catalysts, the fuel, and the temperature that are required. Several types of fuel cells, depending on the compound used as fuel are now under development, each with its own advantages and disadvantages. It seems attractive to use pure hydrogen as fuel because the products of reaction are heat and water/steam in this case. But the direct use of hydrogen as fuel meets some difficulties. Hydrogen does not exist naturally, and its production and distribution require either, as a liquid, low temperature below -240 °C, or, as a compressed gas, high pressure. Moreover, hydrogen has a low energy density in comparison with other hydrogen-containing compounds such as metal hydrides, ammonia, methanol, hydrocarbons and etc. Polymer Electrolyte Membrane (PEM) cells, known as proton exchange membrane cells, are now considered the leading for automotive applications. PEM fuel cell is a thin, flat, multi-layered “sandwich” and is made up of two electrodes (anode and cathode) surrounded by polymer electrolyte [1]. The rise in portable electronics requires the development of miniature fuel cells compatible with standard silicon micro fabrication technology. Composite materials such as inorganic-organic hybrid materials, often including Nafion®as one of components (Nafion-silica, Nafion-borosiloxane etc.) are the next generation of proton conducting membrane [2,3]. The porous silicon wafer filled with acid or Nafion® was developed as a proton conducting membrane [4]. Porous silicon due to its original structural properties desires to be used in fuel cell structures as a solid “electrolyte”. The crystalline structure of porous silicon presents a network of silicon in nano (micro)-regions with an extremely large surface to volume ratio (up to 800m2/cm3). The pore surfaces are covered by Silicon Hydrides (Si-H) and Silicon Oxides (Si-O) bonds along which protons can move [5]. Thus, Porous Silicon (PS) may play the role of proton-conducting electrolyte in porous silicon-based fuel cells. Porous silicon technology can be integrated with the microsystems to be powered [5]. Ammonia provides fuel cells with hydrogen. It contains 17% hydrogen by weight, which can be extracted via thermal catalytic decomposition or electro-oxidation [1]. Although it is toxic, it has a narrower flammable range than hydrogen and is actually considered nonflammable when being transported, whereas hydrogen burns with an invisible flame. Alternatively, ammonia may be oxidized directly in fuel cells without the need for a separate reactor. There are also some significant advantages in terms of storage and transport. Ammonia can be liquefied at room temperature at pressures of 8-10 bar and stored in a similar manner to propane, whereas hydrogen requires expensive cryogenic storage [6]. Ammonia’s energy density is (13.6GJ/m3) much higher than that of hydrogen (3.6GJ/m3) and comparable to that of Compressed Natural Gas (CNG) and methanol, but lower than gasoline and Liquefied Propane Gas (LPG) [7]. Previous research on porous silicon membrane fuel cells has focused on Hydrogen, Hydrogen sulphide, Carbon oxide [6,8,9], Ammonia [10,11] et.al. In this paper is reported the fabrication details, structural, photoluminescence properties of porous silicon and room temperature performance characteristics of new type direct ammonia fuel cells using proton conducting porous silicon membrane and Ag as catalyst.
Experimental Procedure
Porous silicon layers with thickness of 10-40μm and average porosity from 40% to 70% were prepared on n-type monocrystalline (111) Si substrates with resistivity of 0.45Ω∙cm by anodic etching in hydrofluoric-ethanol solution under the white light illumination [6]. For some measurements the PS films were then detached from Si substrate by electro-polishing. The Ag/PS/Si structures were fabricated by evaporation of thin Ag films onto the PS/Si structure at room temperature by using Mo bout in vacuum of 1.3×10−3Pa. The thickness of the deposited silver films (20-40nm) was controlled during the deposition process by Inficon. Electrical measurements of fuel cells were carried out using ammonia solution (NH3:H2O) of different concentrations (0-25%). The composition of the ammonium solution changed with the addition of water. Ammonia and water form ammonium hydroxide on the surface of silver:
NH3+H2O=NH4OH (1)
The crystalline structure of layers was studied by X-Ray Diffraction (XRD) measurements (Bruker XRD D2 PHASER (Germany) Diffractometer in 2θ of 10-70°). Photoluminescence (PL) measurements were performed using PL/PLE/Raman spectrometer (Tokyo Instruments Inc.). The samples were excited by laser beams with a wavelength of 325nm. The current-voltage characteristics, open-circuit voltage (Voc) and short-circuit current density (J) of the Ag/PS/Si cells were measured at room ambient (40% RH) as well as in ammonia solution in measuring cell. The generated by ammonia open-circuit voltage and shortcircuit current between the contacts on Ag and Si substrate were measured directly by digital multi-meter (Thurlby-1503). All of the measurements were performed at room temperatures. The photo sensitive properties of the investigated cells were analyzed by measuring current-voltage characteristics in the dark and in daylight. All the cells exhibited weak photosensitivity and therefore ammonia-stimulated measurements of current-voltage characteristics were performed under daylight conditions.
Results and Discussion
Figure 1 shows the dark current density-voltage characteristic
of Ag/PS/Si cells. It should be noted that the structures didn’t
exhibit any noticeable sensitivity under illumination of120mW/
cm2. Therefore ammonia-stimulated measurements of currentvoltage
characteristics were performed under daylight conditions.
For the XRD study, two types of structures, AS/Ag/PS/Si-n (ASammonia
solution) and Ag/PS/Si-n were fabricated. Both structures
were prepared from the same material and the same thickness (n-
Si ρ=0.45Om.cm, PS d=15 μm, porosity 48%, thickness of Ag film
d=20nm). In case of the first structure three drops of the ammonia
solution (25%) were deposited on the surface of the Ag film. Figure
2 illustrates the diffraction patterns of AS/Ag/PS/Si (curve 1,
blue) and Ag/PS/Si (curve 2, red) structures. XRD patterns of the
sample with a layer of ammonia solution significantly differ from
the patterns of the sample without ammonia solution. Common
to these structures is the observation of patterns around 28° for
(111) plane of porous silicon, 26° and 38° for (210) and (111)
planes of silver, respectively. In the sample containing ammonia
solution on the surface of Ag are seen a few additional patterns,
which are marked by asterisk. The nature of these patterns is not
clear yet, but probably they may be associated with some phases
of Ag-N-H system on the surface of silver. Figure 3 shows the
photoluminescence spectrum of AS/Ag/PS/Si (1-black curve). In
the same figure the PL spectra of AS /Ag/Glass (2-red curve) and
PS/Si (3-green curve) are given for the comparison. As it is seen
from the figure AS/Ag/PS/Si and AS /Ag/Glass structures exhibit
the broadband emission around λ=540nm, meantime the PS/Si
structure without ammonia solution shows just the broadband
emission around λ=700nm, characteristic for porous silicon PL
[12]. The nature of emission band around λ=540nm, as well as the
additional XRD patterns, which are marked by asterisk in Figure 2
is not clear yet. The presence of λ=540nm emission band in AS/Ag/
Glass structure excludes the role of porous silicon in appearance
of this band. The most probable origin can be some compound
on the basis of Ag-N-H system, responsible for this emission. The
well-known Ag (NH3)2 compound should be excluded, so far as the
absorption spectrum of this compound has a maximum at λ=430nm
[13]. Figure 4 shows current density-voltage characteristic of AS/
Ag/PS/Si cell with 25% ammonia solution as fuel (curve 1). It is
seen that AS/Ag/PS/Si cells under ammonia solution produce
electricity (the open-circuit voltage V=0.59V, the short-circuit
current density 134μA/cm2 and maximum power density 47.3μW/
cm2 (curve 2) at room temperature. The Ag/Si structure without
porous silicon layer showed very little sensitivity to ammonia fuel.
Thus, the catalytic layer containing PS plays the main role in the
formation of the electrical parameters of fuel cells. The open-circuit
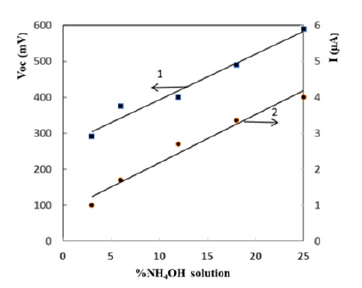
voltage (1) and short-circuit current density (2) of Ag/PS/Si cell
with ammonia for different concentrations at room temperature
are presented in Figure 5. It can be seen that an increase in the
ammonia concentration in the range of 0-25% is accompanied by
an almost linear increase in both the open circuit voltage and the
short-circuit current density. Thus, the following experimental facts
were obtained on research of the electrical characteristics, XRD
data and photoluminescence spectrum of Ag/PS/Si-based fuel cells
operation with ammonium hydroxide solution (ammonia solution)
at room temperature:
A. In the AS/Ag/PS/Si samples containing ammonia solution on
the surface of Ag are seen a few additional patterns, which do
not belong to components of the structure.
B. In contrast to Ag/PS/Si without ammonia solution, AS/Ag/PS/
Si and AS /Ag/Glass structures exhibit the broadband emission
a round λ=540nm, which presumably caused by the formation
of a new phase in Ag-N-H system.
C. The electricity formation was observed for Ag/PS/Si cells
placed in ammonia solution at room temperature. The open
circuit voltage and short-circuit current density of Ag/PS/Si
cells increase with increasing of the ammonia concentration
in the range of 0-25%. The electrical parameters of Ag/PS/Si
cells with ammonia solution as fuel are the peak power density
47.2μW/cm2, the open-circuit voltage 0.59 V and short-circuit
current density 134μA/cm2 at 300K.
Figure 1:Dark current density-voltage characteristic of Ag/PS/Si cells (300K).
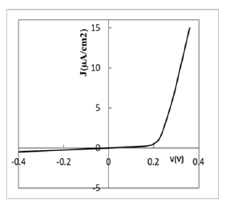
Figure 2:XRD patterns of AS/Ag/PS/Si (1) and Ag/PS/ Si (2) structures.
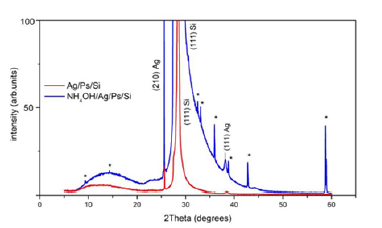
Figure 3:Photoluminescence spectra of AS/Ag/PS/Si (1-black curve), AS/Ag/Glass (2-red curve) and PS/Si (3-green curve) structures.
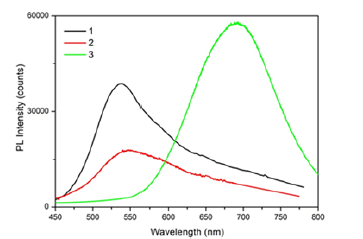
Figure 4:The voltage-current density (1) and power density-current density (2) characteristic of Ag/ PS/Si cells with 25% ammonia solution as fuel (300K).

Figure 5:The open-circuit voltage (1) and shortcircuit current density (2) of Ag/PS/Si cells versus concentration of ammonia (300K).
The mechanism of electricity generation in the investigated cells under the action of an ammonia solution is similar to the previously proposed generation mechanism for the Au/PS/Si element directly exposed to hydrogen or a hydrogen-containing composition as a fuel [8]. Herewith Ag films play the role of the catalytic anode for Ag/ PS/Si cell. The porous silicon acts as proton-conducting membrane and PS/Si interface, which is very imperfect and stressed the role of cathode. Electrochemical reactions proceeding in direct ammonia fuel cell with proton-conducting porous silicon can be expressed as
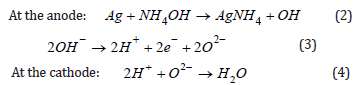
Electrons and protons formed in Ag catalyst films as a result of splitting of hydrogen pass through the external circuit and porous silicon layer, respectively, and reach the cathode (PS/Si interface). Here protons recombine with oxygen ions to produce water molecules.
Conclusion
In this study, fabrication and characterization of Ag/PS/Si ammonia fuel cells with porous silicon membrane and Ag catalyst has been presented. The performance of the cell was measured at room temperature with ammonia solution. The test results confirm that the cell generated an open circuit voltages of 0.59V and a power density of 47μW/cm2 with NH3 solution as fuel. These results demonstrate the feasibility of development of low-cost Ag/ PS-based micro fuel cell for portable electronic application.
References
- Cheddia CD (2012) Ammonia as a hydrogen source for fuel cells. In: Minic D (Ed.), Hydrogen energy challenges and perspectives. Intech, Rijeka, Croatia, pp. 333-362
- Guoxiao X, Zhiguang W, Zenglv W, Wenjie Z, Junli W, et al. (2020) Non-destructive fabrication of nafion/silica composite membrane via swelling-filling modification strategy for high temperature and low humidity PEM fuel cell. Renewable Energy 153: 935-939.
- Suzuki H, Yoshida Y, Mehta MA, Watanabe M, Fujinam T, et al. (2002) Proton conducting borosiloxane solid electrolytes and their composites with nafion®. Fuel Cells 2(1): 46-51.
- Gyoko N, Naohiro I, Takaharu T, Jing Rong Y, Koji T, et al. (2005) Porous silicon as a proton exchange membrane for micro fuel cells. Electrochemistry 73(11): 939-941.
- Dzhafarov TD, Bayramov AB (2017) Porous silicon and solar cells. Handbook of porous silicon. In: Canham L (Ed.), Springer, Germany, p. 1479.
- Dzhafarov TD, Aydin YS (2011) Porous Silicon-based direct hydrogen sulphide fuel cells. J Nanosci Nanotechnol 11(10): 1-4.
- Zamfirescu C, Dincer I (2008) Using ammonia as a sustainable fuel. J Power Sources 185(1): 459-465.
- Dzhafarov TD, Oruc C, Aydin S (2004) Humidity-voltaic characteristics of Au-porous silicon interface. J Phys D Appl Phys 37: 404-408.
- Dzhafarov TD, Aydin YS, Oruc LC (2008) Porous silicon-based gas sensors and miniature hydrogen cells. Jpn J Appl Phys 47(10): 8204 -8207.
- Dzhafarov TD, Aydin YS, Aydin M (2014) Nanoporous silicon-based ammonia-fed fuel cells. Materials Sciences and Applications 5(14): 1020-1026.
- Zhao Y, Setzler BP, Wang J, Nash J, Wang T, et al. (2019) An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule 3(10): 2472-2484.
- Bayramov AI, Mamedov NT, Dzhafarov TD, Aliyeva TN, Ahmadova KN, et al. (2019) Photoluminescence and optical transitions in C60 fullerene thin films deposited on glass, silicon and porous silicon. Thin Solid Films 690: 137566.
- Chen H, Zhang L, Tareque O, Huang J, Wang H, et al. (2013) Microorganism-mediated fabrication and antibacterial performance of Ag/б -Al2O3 Current Nanoscience (10): 271-276.
© 2022 Dzhafarov TD. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






