- Submissions

Full Text
Aspects in Mining & Mineral Science
Using Automotive Paint Sludge as a Binder for Pelletizing Magnetite Ore
Victoria Vaccarezza and Corby Anderson*
Kroll Institute for Extractive Metallurgy, Center for Resource Recovery and Recycling, Colorado School of Mines, USA
*Corresponding author: Corby Anderson, Kroll Institute for Extractive Metallurgy, Center for Resource Recovery and Recycling, Colorado School of Mines, USA
Submission: September 27, 2022;Published: November 23, 2022

ISSN 2578-0255Volume10 Issue2
Abstract
Due to environmental and economic concerns, there has been a large push to investigate recycling applications for automotive paint sludge. Automotive paint sludge is considered a nuisance and hazardous waste material within the automotive industry around the world. The focus of this project was to investigate and develop a recycling application that would employ the use of the automotive paint sludge material as received. The paint sludge was used as a binder for iron ore pellets, typically used for iron- and steelmaking. The development of the type of recycling application conducted during this project was aided by a review of different binders used in iron ore pellets, as well as the various techniques used to recycle automotive paint sludge materials. Two different paint sludge samples were characterized by their organic and inorganic components. It was then determined to be a possible substitute for the standard iron ore pellet binder: bentonite clay. Unlike bentonite, paint sludge has half the concentration of silica and alumina, which are detriments in the ironmaking process. Iron ore pellets were made using magnetite ore, 1.0–3.0 wt.% limestone, 0.0-1.0 wt.% bentonite, and 0.0-1.0 wt.% paint sludge from two different paint shops. The pellet recipes were evaluated via a statistical analysis software where low, middle and high material weight percentages were output to ensure optimized results. The iron ore pellets with paint sludge material as a binder were then tested for their standard physical and chemical properties to make sure they were comparable to pellets made with bentonite clay. It was concluded via the physical and chemical property tests that iron ore pellet made with paint sludge material with this patent pending technology were just as capable of withstanding typical transportation and handling used in the iron- and steelmaking industries.
Keywords:Paint sludge; Steelmaking; Iron ore pellets; Limestone; Bentonite
Introduction
Automotive paint sludge is a by-product of overspray in the painting booths. Paint sludge is either water-based or solvent-based, however, both types contain a combination of inorganic and organic material. The designation of waterborne or solvent-based depends on the type of paint material, i.e., if the paint is contained in a water base, it is waterborne. Both types of paint sludge consist of uncured polymer resins, metal-containing pigments, curing agents, flotation agents, de-tackifiers and water. Waterborne paint sludge has been promoted as the environmentally friendly option in comparison, but the entire vehicle painting process does still rely on organic materials. It is for this reason the generation of automotive paint sludge, regardless of its base, is considered one of the largest contributors of hazardous waste in the automotive industry [1]. However, the new millennia also brought new environmental concerns across all industries, as well as increases in fees for the storage of hazardous material. In response, the automotive industry has become the leader in promoting zero waste [2]. This has also led many, if not all, automotive companies to seek other techniques for recycling their paint sludge. Previous research methods include attempting to extract valuable metals or utilizing them for construction materials. Iron ore pellets are used in the steel making process with bentonite clay as the current industry standard binder. This investigation seeks to develop comparable iron ore pellets with a paint sludge binder by employing similar testing techniques used for traditional iron ore pellets [3,4].
In addition to the experimental results presented, the implications of these results will be discussed as they relate to the two major ironmaking methods: blast furnace and direct reduction. The blast furnace is considered an indirect reduction of iron in combination with a converter, while direct reduction produces Direct Reduced Iron (DRI) [5]. It is critical to examine how the use of a different iron ore pellet binder affects the ironmaking process in both the blast furnace and direct reduction processes since each has differences in feed requirements and allowances.
Materials and Methods
A 2n half-factorial design was chosen for this project due to
insufficient raw materials for the experiments. “n” is the number
of controllable factors included in the experiment by the user. The
controllable factors included weight percent addition of paint
sludge, weight percent addition of bentonite, weight percent
addition of limestone, temperature of induration and time of
induration. The values of each factor were chosen based on current
industry values, the literature review as well as the project’s
experimental need. Inputting the information into Stat-Ease, the
following DOE was produced shown in Table 1. As seen in Table
2, a total of 19 runs were done for the first batch of paint sludge. A
second sample was labeled as batch 2 of paint sludge and the halffactorial
design is presented in Table 3. This presented another set
of 19 runs, totaling 38 between both samples of paint sludge. Runs
1-19 represent batch 1, while runs 20-38 represent batch 2 [6-10].
The responses for this study are shown in Table 4 for all the runs
done. Based on the information from the design of experiments,
a variety of magnetite, paint sludge, bentonite and limestone
combinations were given. The combinations include:
A. 0 wt.% paint sludge, 0 wt.% bentonite, 0 wt.% limestone, and
100 wt.% magnetite ore.
B. 0 wt.% paint sludge, 1 wt.% bentonite, 0 wt.% limestone, and
99 wt.% magnetite ore.
C. 0 wt.% paint sludge, 0 wt.% bentonite, 3 wt.% limestone, and
97 wt.% magnetite ore.
D. 0 wt.% paint sludge, 1 wt.% bentonite, 3 wt.% limestone, and
96 wt.% magnetite ore.
E. 0.5 wt.% paint sludge, 0.5 wt.% bentonite, 1.5 wt.% limestone,
and 97.5 wt.% magnetite ore.
F. 1 wt.% paint sludge, 0 wt.% bentonite, 0 wt.% limestone, and
99 wt.% magnetite ore.
G. 1 wt.% paint sludge, 1 wt.% bentonite, 0 wt.% limestone, and
98 wt.% magnetite ore.
H. 1 wt.% paint sludge, 0 wt.% bentonite, 3 wt.% limestone, and
96 wt.% magnetite ore.
I. 1 wt.% paint sludge, 1 wt.% bentonite, 3 wt.% limestone, and
95 wt.% magnetite ore.
Table 1:Design of experiments for iron ore pellets with paint sludge binder.
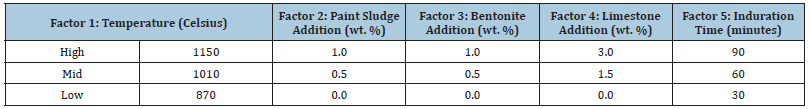
Table 2:Half- factorial design order for batch 1 of paint sludge.
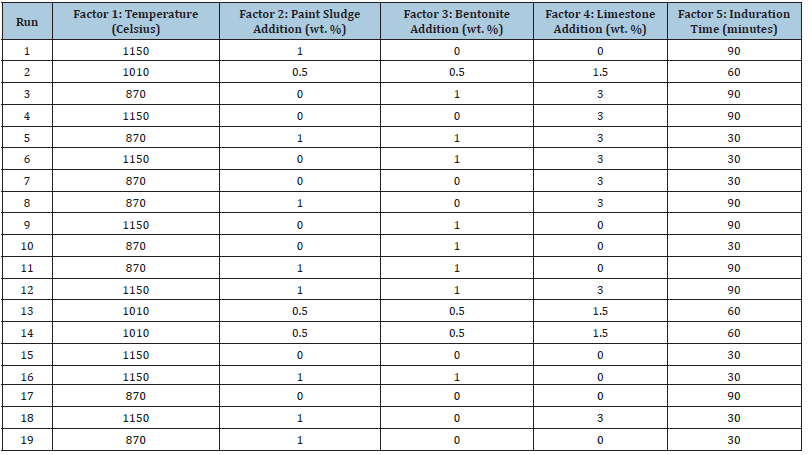
Table 3:Half-factorial design order for batch 2 of paint sludge.
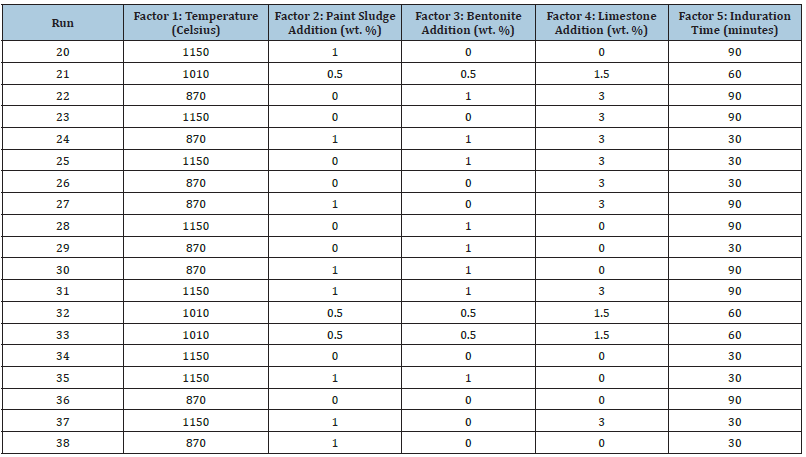
Table 4:Responses for half-factorial design order.

First, the materials were combined and mixed in a Tupperware container. A spray bottle is also used to moisten the materials. About 15-25 sprays per 100 grams of total material, which equals to about 1-1.5mL of water for the specific spray bottle used in this project. This provided just enough water to wet the material but not enough to overly saturate it. A small amount of material was then taken and rolled into a sphere between the palms of the hand as shown in Figure 1. As described in the literature review, pellets used in the DRI and blast furnace ironmaking processes are between 9 and 16mm in diameter and the same diameter range was used in this project as well. About 15-20 green pellets were made from 100 grams of material. This procedure was repeated for all the combinations of paint sludge, bentonite, limestone and magnetite ore provided above. After the green pellets were made, they were dried in a furnace for 12 hours at 100 °C. After drying, the pellets are considered dry pellets and placed in a furnace for induration. Based on the design of experiments, specific combinations were fired at different temperatures and times [11-15]. Pellets with the following combinations of materials were fired at 870 °C for 30 minutes:
Figure 1:Combination of materials: paint sludge (gray), magnetite ore (black) and limestone (white).

a) 0 wt.% paint sludge, 0 wt.% bentonite, 3 wt.% limestone, and
97 wt.% magnetite ore.
b) 0 wt.% paint sludge, 1 wt.% bentonite, 0 wt.% limestone, and
99 wt.% magnetite ore.
c) 1 wt.% paint sludge, 1 wt.% bentonite, 3 wt.% limestone, and
95 wt.% magnetite ore.
d) 1 wt.% paint sludge, 0 wt.% bentonite, 0 wt.% limestone, and
99 wt.% magnetite ore.
Pellets with the following combinations of materials were fired
at 870 °C for 90 minutes:
A. 0 wt.% paint sludge, 1 wt.% bentonite, 3 wt.% limestone, and
96 wt.% magnetite ore.
B. 0 wt.% paint sludge, 0 wt.% bentonite, 0 wt.% limestone, and
100 wt.% magnetite ore.
C. 1 wt.% paint sludge, 0 wt.% bentonite, 3 wt.% limestone, and
96 wt.% magnetite ore.
D. 1 wt.% paint sludge, 1 wt.% bentonite, 0 wt.% limestone, and
98 wt.% magnetite ore.
The next set of pellets were fired at 1010 °C for 60 minutes for
the following combination of materials:
A. 0.5 wt.% paint sludge, 0.5 wt.% bentonite, 1.5 wt.% limestone,
and 97.5 wt.% magnetite ore.
Pellets with the following combination of materials were fired
at 1150 °C for 30 minutes:
a) 0 wt.% paint sludge, 1 wt.% bentonite, 3 wt.% limestone, and
96 wt.% magnetite ore.
b) 0 wt.% paint sludge, 0 wt.% bentonite, 0 wt.% limestone, and
100 wt.% magnetite ore.
c) 1 wt.% paint sludge, 1 wt.% bentonite, 0 wt.% limestone, and
98 wt.% magnetite ore.
d) 1 wt.% paint sludge, 0 wt.% bentonite, 3 wt.% limestone, and
96 wt.% magnetite ore.
Finally, pellets with the following combination of materials
were fired at 1150 °C for 90 minutes:
A. 1 wt.% paint sludge, 0 wt.% bentonite, 0 wt.% limestone, and
99 wt.% magnetite ore.
B. 0 wt.% paint sludge, 0 wt.% bentonite, 3 wt.% limestone, and
97 wt.% magnetite ore.
C. 0 wt.% paint sludge, 1 wt.% bentonite, 0 wt.% limestone, and
99 wt.% magnetite ore.
D. 1 wt.% paint sludge, 1 wt.% bentonite, 3 wt.% limestone, and
96 wt.% magnetite ore.
Test Methods
The use of iron ore pellets for the production of iron and
steel is so widely used in both blast furnaces and DRI process,
it is important to make sure the pellets will meet performance
standards. There are several quality testing methods for acceptance
of suitable pellets. Iron ore can originate from various locations
and different companies may be using a variety of types of binders
and additives. Because of this, various quality measurements have
become standardized for quality control of the iron ore pellets. The
physical methods include [16-25].
a) Drop number/impact: the purpose of this test is to estimate
how well the pellet can withstand being dropped during
handling. This is done by dropping the pellet (green/dry/fired)
from a specific height onto a steel plate until fracture, counting
the number of times it survived before fracture. Typical values
are 4-7 for green pellets and more than 8 times for fired pellets.
There is no standardized version of this method.
b) Compressive strength: this tests the ability of the pellets
to handle a compressive load without fracture. It is done by
applying a compressive load to the pellet at a constant rate
until the pellet fractures. Dry and indurated pellets are usually
tested. Acceptable fracture loads are 250-300kg/pellet for
indurated pellets. The standardized method is ISO 4700.
c) Bulk density: this determines the bulk density of the iron ores,
indicating how much weight of a material may be packed per
unit area. This test is done on fired pellets and acceptable
values are between 2000-25000kg/m3. The standardized
method is ISO 3852.
d) Apparent density: This estimates the apparent density and
water adsorption of the DRI by immersion in water, giving
porosity of the pellets. This is important for the reducibility of
the pellets. The acceptable values are 3000-5000kg/m3. The
standardized method is ISO 15986.
e) Cluster index: This method tests for the tendency of the
indurated pellets to form clusters during the reduction in the
shaft furnace. A cluster index greater than 20 is preferred. The
standardized method is ISO 11256.
f) Tumble index: This tests the friability of the fired pellets which
is important during transportation and handling. Acceptable
values are 93-96%, +6.3mm. The standardized method is ISO
3271.
g) Abrasion index: Similar to the tumble index, this method
estimates the likelihood of the fired pellets generating fines
during handling. Fine generation in the blast furnace can cause
issues with gas permeability through the furnace, leading to
operational issues. An abrasion index less than 5%, -0.5mm is
preferred. The standardized method is ISO 3271.
h) Reducibility: Indurated pellets are tested to determine the
extent of metallization under reducing conditions. Acceptable
values are between 1.2 and 1.3. The standardized method is
ISO 11257.
i) Reduction-disintegration index: Indurated pellets are tested
for an estimate of fines generated during reduction. Typical
values are 1.5-2.0%, -3.15mm. The standardized method is ISO
11257.
j) Swelling index: Indurated pellets are tested for the percent
in volume increase at different temperatures. This method is
mainly used for blast furnace pellets. The typical value is 20%.
The standardized method is ISO 4698.
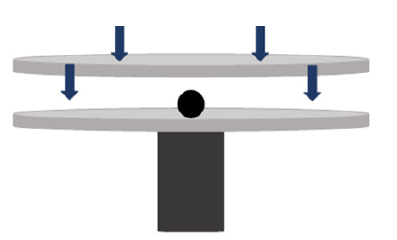
The pellets were dropped from one meter, measured using a meter stick, onto a steel plate until counting the number of drops until they fracture. Figure 2 shows a schematic diagram of the drop test setup. From each combination set, 5 pellets were tested from batch 1 and batch 2 resulting in a total of 90 dry pellets. The average value of each set of 5 was recorded as the drop number for that specific material combination. 5 fired pellets from each of the material combinations for 870 °C for 30 and 90 minutes, 1010 °C for 60 minutes, and 1150 °C for 30 and 90 minutes, were tested and the average drop number was recorded. This was done for batch 1 and batch 2 pellets, resulting in a total of 170 fired pellets tested (Figure 3). This compression test was done for all the dry pellets made from each of the 9 material combinations. From each combination set, 5 pellets were tested resulting in a total of 90 dry pellets. The average value of each set of 5 was recorded as the crushing strength for that specific material combination. Since the fired pellets were indurated at different temperatures and times, 5 fired pellets from each of the material combinations for 870 °C for 30 and 90 minutes, 1010 °C for 60 minutes, and 1150 °C for 30 and 90 minutes, were tested and the average was recorded. This was done for batch 1 and batch 2 pellets, resulting in a total of 170 fired pellets tested.
Figure 2:Schematic representation of drop test.
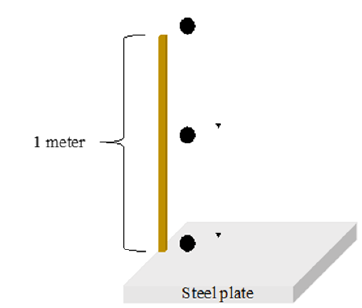
Figure 3:Schematic diagram of compressive plates and pellet.
The procedure used to calculate the apparent porosity of fired pellets is the ASTM C20-00(2015) Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. Apparent porosity is the percentage ratio of the void space in a sample to the total bulk volume of the sample. Equation 3 describes the calculation of apparent porosity, Wsat is the weight of saturated sample, Wdry is the weight of the sample in air, and Wsus is the weight of sample suspended in water.
Figure 4:Schematic diagram on saturation process.
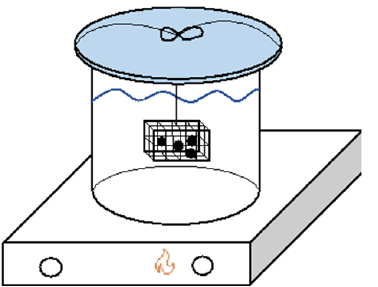
Determination of the dry weight is done by weighing the pellets with a scale in air, however, the pellets must have been dried in a furnace above 105 °C. Since these pellets were fired, they were just weighed after induration. Four pellets were placed in the metal cage at a time. The metal cage was suspended from a watch glass. The cage was dropped into a beaker of boiling water and kept there for two hours. The pellets did not touch the bottom of the beaker and the watch glass did not allow for a lot of evaporation, so the metal cage was suspended in water for the duration of the two hours. A representation of the setup is shown in Figure 4. While the pellets are boiling, the air in the pores expands and are partially substituted by water. While the water and pellets are cooling, some of that water may stay within the pores, but the air that remained in the pore’s contracts, causing a negative pressure or vacuum. This vacuum is the driving force for the fluid to enter the pores. After the pellets were boiled, they were brought to room temperature while still suspended in the beaker, completely covered in water for about twelve hours. After twelve hours, they were weighed and recorded as the suspended weight. The saturation weight is determined after the suspended weight by blotting the pellets to remove any excess water on the surface of the pellets. Reduction testing was conducted on the pellets. Figure 5 shows a representation of the tube furnace and sample setup.
Figure 5:Tube furnace and sample setup for reduction tests.
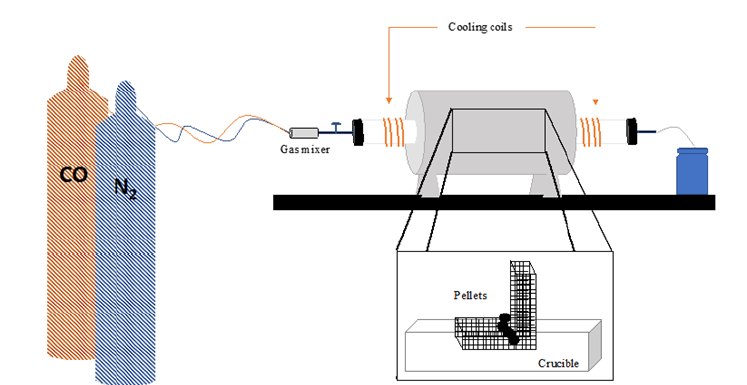
The procedure involved reducing the pellets determined by the factorial design for batch 1 and batch 2 pellets. Four to five pellets at a time were placed on the metal mesh and then into the tube furnace. The furnace was heated to 950 °C with 50-60mL/ min of nitrogen gas flowing. Once the furnace reached the desired temperature, the carbon monoxide gas was opened and increased to a flow rate of 150mL/min. The nitrogen gas was also increased to 100mL/min. Both gases were kept at this rate for one hour. The carbon monoxide was slowly shut off and the nitrogen gas flow decreased to 50mL/min while the furnace was cooled down. After the furnace was cooled, the pellets were taken out, weighed and their diameter’s measured and recorded. A total of 38 pellets were reduced by this procedure.
Results
Table 5 shows the crushing strength values in increasing order
for dry and fired pellets made from the paint sludge sample from
batch 1. As seen in the table, the biggest indicator of increased
cold crushing strength is increased temperature. Most of the
values of cold crushing strength above 250kg/pellet were seen in
pellets indurated at 1010 °C and 1150 °C. They were also left at
that induration temperature for 60 minutes or longer. The type of
pellets that pass the 250kg/pellet threshold are the following:
A. 1 wt.% paint sludge, 1 wt.% bentonite, and 0 wt.% limestone
B. 1 wt.% paint sludge, 1 wt.% bentonite, and 3 wt.% limestone
C. 0 wt.% paint sludge, 1 wt.% bentonite, and 3 wt.% limestone
D. 0.5 wt.% paint sludge, 0.5 wt.% bentonite, and 1.5 wt.%
limestone
E. 1 wt.% paint sludge, 0 wt.% bentonite, and 3 wt.% limestone
F. 0 wt.% paint sludge, 0 wt.% bentonite, and 0 wt.% limestone
G. 1 wt.% paint sludge, 0 wt.% bentonite, and 0 wt.% limestone
Some of this material combinations can also be found in Table 5 to not pass the crushing strength threshold and that is due to low temperature of 870 °C and short induration time. The question driving this research was if waterborne automotive paint sludge could replace all or some of the bentonite additions made when producing iron ore pellets. Based on the data in Table 5, 0.5PS_0.5B_1.5LS, 1PS_0B_3LS, and 1PS_0B_0LS pellet types prove this to be possible with the paint sludge from batch 1. The pellet type 0PS_0B_0LS is interesting because at 1150 °C for 30 minutes, it does not pass the crushing strength threshold, however, kept at that temperature for 90 minutes, the cold crushing strength increases from 249 to 254. This may be due to the small amounts the bentonite phase found in the AMICS analysis of the magnetite ore sample. Figure 6 shows another representation of how different temperatures affect the cold crushing strength of pellets of the same material combinations. In all pellet type combinations, an increase in temperature increases the cold crushing strength. Pellet type 0.5PS_0.5B_1.5LS only shows one value of cold crushing strength because it was solely indurated at 1010 °C, while the other pellet types were indurated at both 870 °C and 1150 °C.
Table 5:Batch 1 pellet dry and cold crushing strength.
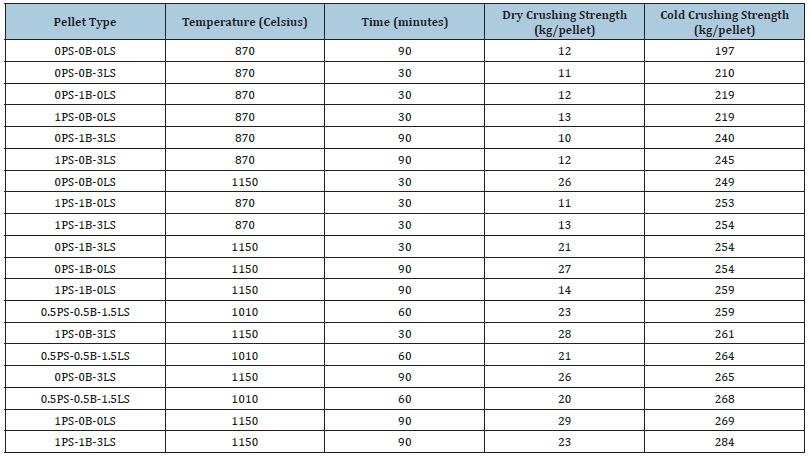
Figure 6:Cold crushing strength (CCS) for batch 1 pellets.
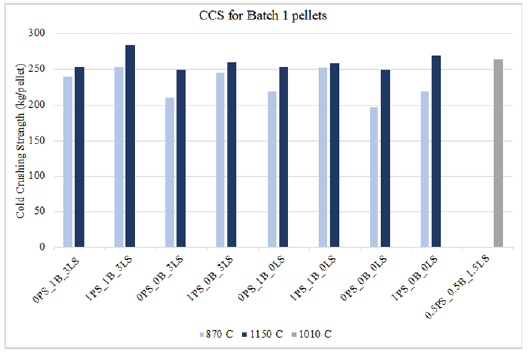
A comparison between the crushing strength of dry and fired pellet types are shown in Figures 7 & 8, for pellets fired at 870 °C and 1150 °C, respectively. These figures highlight the differences in chemical and physical strengthening mechanisms occurring while the pellet is exposed to different temperatures. A dry pellet is dried at 100 °C and is considered more fragile than green pellets because the capillary forces that held the green pellets together are now gone due to water evaporation. The water evaporation leaves the dry pellets mechanically weakened, and it may be argued that the plasticity behavior once present in the green pellets is now gone. As shown in Figures 7 & 8, the dry crushing strength values are significantly lower compared to those fired at 870 °C and 1150 °C. However, once the pellets have been indurated, they do become mechanically stronger but for different reasons at the two temperatures. Pellets indurated at 870 °C become denser due to the phase change occurring during the oxidation from magnetite to hematite. On the other hand, pellets indurated at 1150 °C are shown to have higher crushing strength values because of sintering effects. Sintering in iron ore pellets occurs above 1000 °C and is a major contributor to the strength of iron ore pellets.
Figure 7:Crushing strength of dry vs. fired batch 1 pellets at 87 °C.
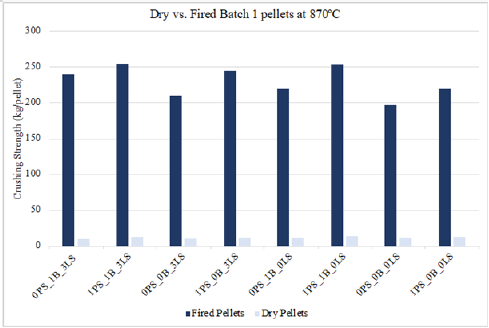
Figure 8:Crushing strength of dry vs. fired batch 1 pellets at 115 °C.
Crushing strength test results for batch 2 pellets
Table 6 shows the dry and cold crushing strength values for
both dry and fired pellets in increasing cold crushing strength
values. As mentioned before, increased temperature is shown to
be the biggest indicator of increased pellet cold crushing strength.
The same 250kg/pellet crushing strength threshold was applied
to Table 6 and it shows the following pellet type combinations as
passing that value:
a) 0.5 wt.% paint sludge, 0.5 wt.% bentonite, and 1.5 wt.%
limestone
b) 1 wt.% paint sludge, 1 wt.% bentonite, and 0 wt.% limestone
c) 1 wt.% paint sludge, 0 wt.% bentonite, and 0 wt.% limestone
d) 1 wt.% paint sludge, 0 wt.% bentonite, and 3 wt.% limestone
e) 0 wt.% paint sludge, 1 wt.% bentonite, and 0 wt.% limestone
f) 1 wt.% paint sludge, 1 wt.% bentonite, and 3 wt.% limestone
Some of these pellet type combinations are also seen in Table 6 to have crushing strength values below 250kg/pellet, but those are due to their low induration temperature of 870 °C. All the pellets that had crushing strength values over 250kg/ pellet were indurated at 1010 °C or 1150 °C for 60 minutes or 90 minutes. Figure 9 is another representation of this as it shows the cold crushing strength increase with increased temperature for pellets of the same material combination. Figure 10 & 11 show the crushing strength for dry and fired pellets indurated at 870 °C and 1150 °C, respectively. As mentioned in the previous section, differences in dry and cold crushing strength are most likely due to water evaporation in the dry pellets and the formation of denser hematite pellets at 870 °C. Of course, as the temperature increased to 1150 °C, sintering effects begin to substantially strengthen the pellets compared to what is seen at 870 °C.
Table 6:Batch 2 pellets dry and cold crushing strength
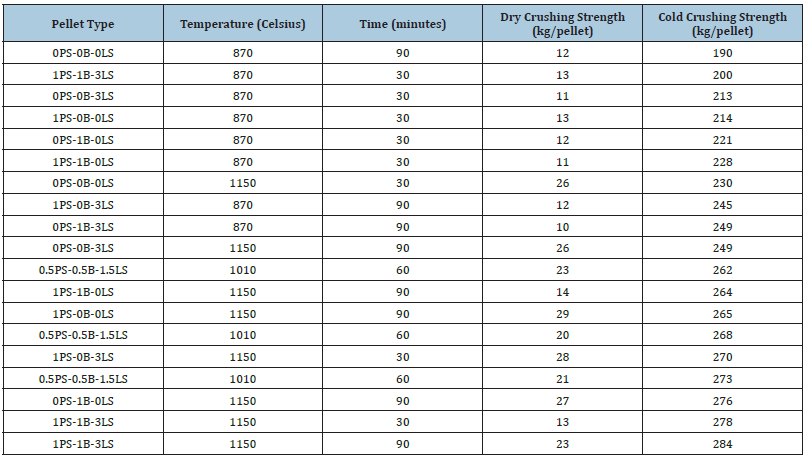
Figure 9:Cold crushing strength for batch 2 pellets.
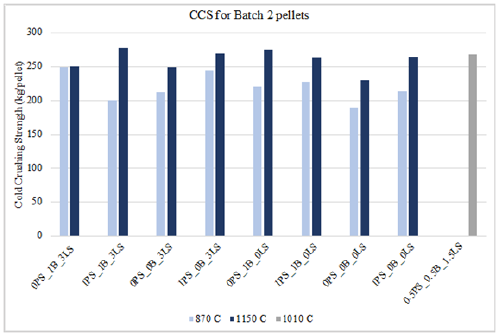
Figure 10:Crushing strength for dry vs. fired batch 2 pellets at 87 °C.
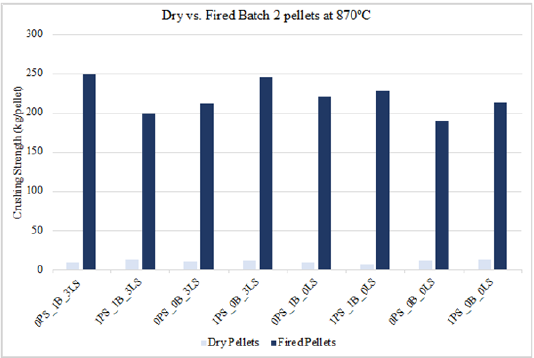
Figure 11:Crushing strength for dry vs. fired batch 2 pellets at 115 °C.
Drop test results for batch 1 pellets
A drop or impact test is conducted on the pellets as another measure in determining how well the pellets will withstand transportation and handling. Most literature data suggest drop number values over 8 as durable for fired pellets. The drop test differs from the compression test in that the pellet is free falling and only one part of the pellet is impacted. Table 7 shows the drop test numbers for dry and fired pellets in increasing drop number for fired pellets. All the pellet types that passed the 8 drop number value were pellets indurated at a high temperature: 1010 °C and 1150 °C. In Table 7 we see that some of the pellets that pass the drop number threshold were pellets with no additions of bentonite, and only consisted of paint sludge, magnetite, and/or limestone (e.g., 1PS_0B_3LS and 1PS_0B_0LS) or half of the bentonite addition was replaced with paint sludge (0.5PS_0.5B_1.5LS). It can be concluded then that magnetite ore pellets substituted completely or partially with automotive paint sludge still display impact values comparable to pellets with bentonite clay. Figure 12 shows the drop test values for fired pellets made from batch 1 paint sludge material for induration temperatures 870 °C, 1010 °C, 1150 °C. This graph shows the temperature dependence again, implying the importance of sintering in the strengthening of iron ore pellets. The pellet type 0PS_0B_0LS does not show any promising impact values, most likely due to the lack of bentonite and paint sludge materials.
Table 7:Drop number for dry and fired batch 1 pellets.
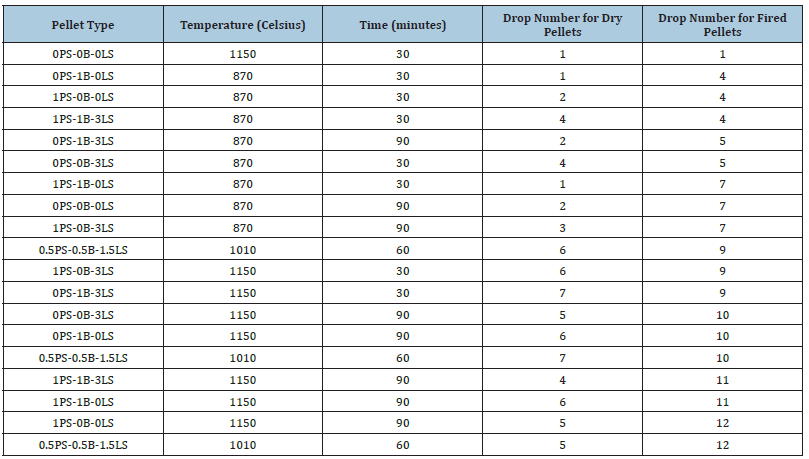
Figure 12:Drop test numbers for fired batch 1 pellets.
Drop test results for batch 2 pellets
As discussed in the section before, the same drop number of 8 is considered the durable threshold for suitable iron ore pellets. Most of the pellets that pass this threshold value are pellets indurated at 1010 °C and 1150 °C where sintering effects significantly increase the strength and hardness of a pellet. Table 8 shows pellet type combinations that do not include the addition of bentonite or the partial addition of bentonite (e.g., 0.5PS_0.5B_1.5LS and 1PS_0B_0LS, and 1PS_0B_3LS), indicating the paint sludge material as a suitable complete or partial substitution. Figure 13 displays the drop number values for fired pellets from batch 2 paint sludge material indurated at 870 °C, 1010 °C and 1150 °C. While not all the fired pellets drop in number, values pass the 8 drop threshold, it does show how the temperature dependence; the pellets withstand more drops as their induration temperature increases. The pellet type 0PS_0B_0LS cannot withstand numerous drops due to the lack of bentonite and paint sludge material to strengthen the pellets. Finally, Figure 14 shows a comparison between drop test values between batch 1 and batch 2 fired pellets. Pellets indurated above 1000 °C are shown to withstand multiple drops and are considered durable enough for handling and transportation in a manner typical of an industrial pelletizing plant (Tables 9 & 10).
Table 8:Drop number for dry and fired batch 1 pellets.
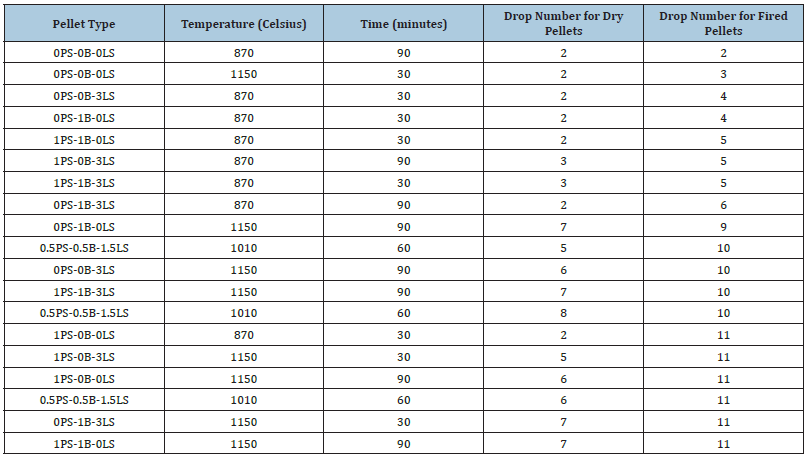
Figure 13:Drop test numbers for fired batch 2 pellets
Figure 14:Drop number versus temperature of induration for pellets of batch 1 and 2.
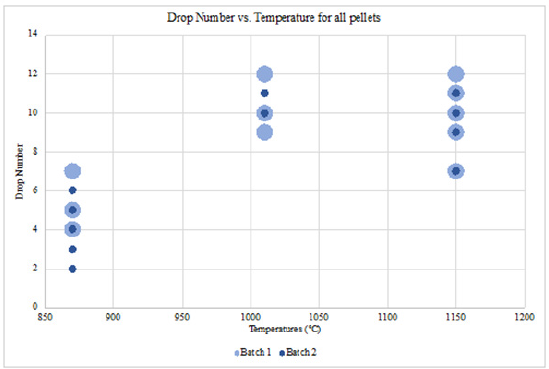
Table 9:Apparent porosity and percent reduction of batch 1 pellets.
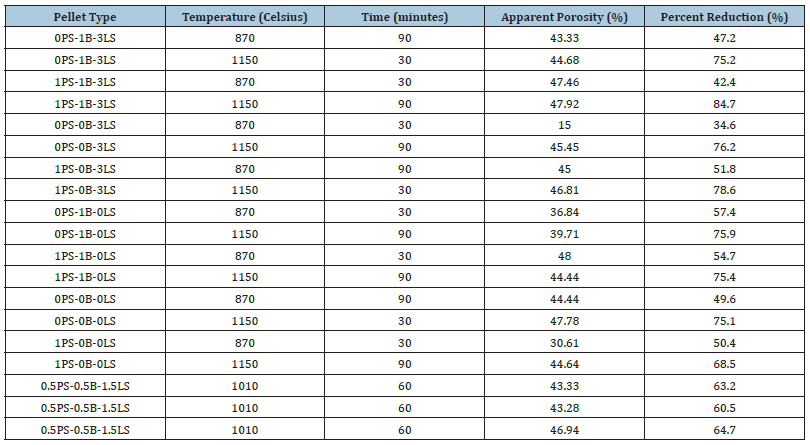
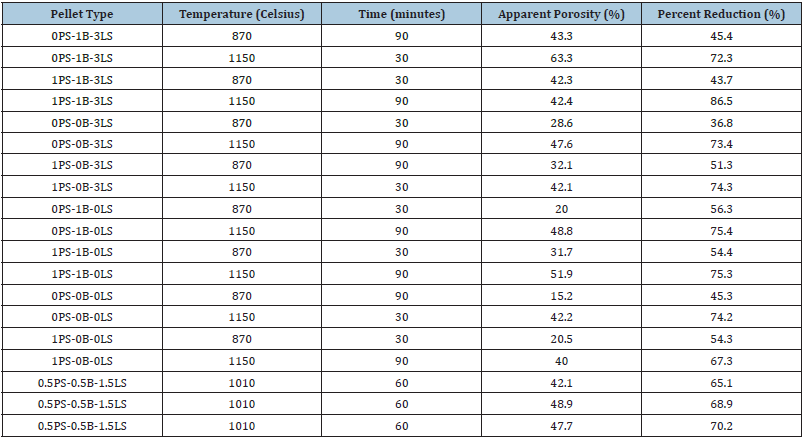
Table 10:Apparent porosity and percent reduction of batch 2 pellets.
Conclusion
The goal of this research project was to investigate the effectiveness of using waterborne automotive paint sludge as a binder for making iron ore pellets. The typical binder used for iron ore pellets is bentonite clay, however, due to the high costs and low availability, alternatives to bentonite clay have been researched for years. Bentonite clay provides the iron ore pellets with an increase in silica and alumina, which are considered detrimental in the downstream ironmaking process. Too much silica and alumina increase the acidity of the slag while it is being processed in a blast furnace. Increased slag acidity increases the viscosity of the slag and the difficultly in tapping the slag. This is not a superficial issue that can be easily solved since the operations of a blast furnace cannot be turned on and off easily. In addition to the issues associated with the blast furnace, DRI pellets should also not contain high concentrations of silica or alumina because there is not a lot of room for impurities or to resolve the impurities within the DRI process.
Many of the alternatives investigated seek to eliminate or limit silica and alumina. Organic materials, such as polymers, have been intensely studied as an alternative binder to bentonite clay because their long chains can hold together pellets similar to bentonite. Because they are organic material, they may also add heat to the BF or DRI process, aiding in the energy costs. However, organic materials are very expensive, and, in many cases, the cost associated with creating an organic binder for the amount of pellets used cannot be justified. This is precisely why automotive paint sludge should be considered in a technical and economical alternative binder. It not only contains a substantial amount of organic material, including polymers, and low amount of silica and alumina, but is also economically favorable for automotive companies. Bentonite resources and costs are also a driver for alternate binders. Deposits in Wyoming where a bed of volcanic ash reacted with water are the best-grade sodium bentonites in North America. It costs about $0.025/lb ($0.055/kg) for production and while this cost is not expensive, high grade sodium bentonite is in great demand.
Recycling of automotive paint sludge is an important topic of late because many automotive companies are trying to reduce their waste in landfills. The paint sludge consists of organic and inorganic material, in this case, mostly titanium dioxide with significantly lower percentages of silica and alumina compared to bentonite. To test how well the paint sludge could substitute as a binder compared to bentonite, iron ore pellets were made of typical industrial standard with and without bentonite. These pellets were subject to a crushing strength test, a drop test, determination of apparent porosity and percentage reduction. All these properties are considered important for the durability and efficiency of the iron ore pellets. It was determined that iron ore pellets where the bentonite was completely and partially replaced with paint sludge materials from two different paint shops, had comparable cold crushing strength values above 250kg/pellet as per industry standards. In addition to the cold crushing strength values, the pellets also withstood multiple drops above 8 drops which is also typical of industry standards. Apparent porosity and percent reduction were also determined to have suitable values showing that the paint sludge can replace bentonite as a binder. Bentonite resources and costs are also a driver for alternate binders. Deposits in Wyoming where a bed of volcanic ash reacted with water are the best-grade sodium bentonites in North America. It costs about $0.025/lb ($0.055/kg) for production and while this cost is not expensive, high grade sodium bentonite is in great demand.
Acknowledgement
The authors acknowledge and appreciate the support for this research from the National Science Foundation, General Motors Corporation, Heritage Environmental and the Center for Resource Recovery and Recycling.
References
- Salihoglu G, Salihoglu NK (2016) A review on paint sludge from automotive industries: Generation, charateristics and management. Journal of Environmental Management 169: 223-235.
- Friess S (2017) Carmakers try to keep waste out of the ground as well as the air. The New York Times, USA.
- (2018) United States Environmental Protection Agency. How communities have defined zero waste.
- Simscale (2021) What is FEA (Finite Element Analysis)?
- ArcelorMittal (2016) Steel making process.
- Lu L, Pan J, Zhu D (2015) Quality requirements of iron ore for iron production. Iron Ore: Mineralogy, Processing and Environmental Sustainability pp. 475-504.
- Battle T, Srivastava U, Kopfe J, Hunter R, McClelland J (2014) Chapter 1.2 The direct reduction of iron. Treatise on Process Metallurgy: Industrial Process, Elsevier, pp. 89-176.
- Halt JA, Kawatra SK (2014) Review of organic binders for iron ore agglomeration. Minerals and Metallurgical Processing 31(2): 73-94.
- Ball D (1973) Agglomeration of iron ores. New York: American Elsevier, US.
- Pietsch WB (2002) Agglomeration processes: Phenomena, technologies, equipment. Weinheim: John Wiley & Sons, US.
- Rumpf H (1962) The strength of granules and agglomerates. Agglomeration Proceedings of the First International Symposium on Agglomeration, Philadelphia, US, pp. 379-418.
- De Moraes SL, de Lima J, Ribeiro T (2018) Iron ore pelletizing process: An overview. Iron Ores and Iron Oxide Materials.
- Sastry K, Fuerstenau DW (1973) Mechanisms of agglomerate growth in green pelletization. Powder Technology 7(2): 97-105.
- Meyer K (1980) Pelletizing of iron ores. Berlin: Springer Verlag, Germany.
- Zhu D, Pan J, Lu L, Holmes RJ (2015) Iron ore pelletization. Iron Ore, Elsevier, pp. 435-473.
- Heikkila A, Iljana M, Bartusch H, Fabritius T (2020) Reduction of iron ore pellets, sinter, and lump ore under simulated blast furnace conditions. Steel Research International 91(11).
- Schmitt J (2005) A method for improving the process and quality of iron ore pellets made with organic binders. 78th Annual Minnesota Section of SME meeting & 66th Annual University of Minnesota Mining Symposium, Duluth, US.
- ASTM E382-20 (2020) Standard test method for determination of crushing strength of iron ore pellets and direct-reduced iron. West Conshohocken: ASTM International, US.
- ASTM C20-00 (2015) Standard test methods for apparent porosity, water absorption, apparent specific gravity, and bulk density of burned refractory brick and shapes by boiling water. West Conshohocken: ASTM International, US.
- ISO 4700 (2015) Iron ore pellets for blast furnace and direct reduction feedstocks-Determination of the crushing strength. International Organization for Standardization.
- ISO 3852 (2007) Iron ores for blast furnace and direct reduction feedstocks-Determination of bulk density. International Organization for Standardization.
- ISO 11256 (2015) Iron ore pellets for shaft direct-reduction feedstocks-Determination of the clustering index. International Organization for Standardization.
- ISO 3271 (2015) Iron ores for blast furnace and direct reduction feedstocks-Determination of the tumble and abrasion indices.
- ISO 11257 (2015) Iron ores for shaft direct reduction feedstocks-Determination of the low-temperature reduction, disintegration index and degree of metallization.
- ISO 4698 (2007) Iron ore pellets for blast furnace feedstocks-Determination of the free-swelling index.
© 2022 Corby Anderson. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






