- Submissions

Full Text
Aspects in Mining & Mineral Science
Characterization of the Hoidas Lake Rare Earth Deposit
Corby G Anderson*
Kroll Institute for Extractive Metallurgy, Colorado School of Mines, USA
*Corresponding author: Corby G Anderson, Kroll Institute for Extractive Metallurgy, Colorado School of Mines, Golden, Colorado, USA
Submission: September 28, 2022;Published: October 28, 2022

ISSN 2578-0255Volume10 Issue2
Abstract
Hoidas Lake lies in the Northern Rae Geological Province, in the general vicinity of many of Saskatchewan’s large uranium mines. The mineralogy of the Hoidas Lake rare-earth deposit differs from most other such deposits in that it is hosted in equal abundance in veins containing apatite and allanite mineral groups. Hoidas Lake also differs from other deposits in that it contains a significant amount of heavy rare-earth elements, such as dysprosium [1-5]. This abundance of heavy Rare Earth Elements (REE’s) is significant, as there is a growing demand for the heavier rare earths in high-tech manufacturing (such as the use of dysprosium in the manufacturing of hybrid car components).
Keywords:Hoidas lake; Rare earth elements; Characterization
Introduction
Research was provided for assessing ten (10) boxes of core samples provided by Great Western Minerals. This provided a basis for further metallurgical testing which included beneficiation and hydrometallurgical rare earth separation and recovery [6-12]. The following work was done on the ten (10) core samples;
a. Core and bulk composite ore preparation
b. Mineralogical examinations
c. X-Ray diffraction
d. ICP elemental analysis
e. Sulfur and carbon analysis
f. SEM/EDX analysis
g. Abrasion indices of a bulk blended composite sample
h. Bond grindability index of bulk bended composite sample
i. Reporting
Mineralogical Examinations
Ten specimens from the boxed core were selected for thin sections. An effort was made to obtain a representative suite of the various lithologies represented by this core. Preliminary petrographic examination was made of these thin sections accompanied by SEM and EDX analyses of some specimens. The following observations are the most important ones to be considered in carrying out subsequent beneficiation tests on these cores [13-20].
a. The report done by the Mineral Exploration Branch of the Saskatchewan Research Council is excellent. They did a very detailed and accurate petrographic analysis of their specimens. These descriptions together with their chemical analyses are a good starting point for beneficiation tests on the cores.
b. Apart from ilmenite and scapolite we observed all of the minerals described in their report. The carbonate that they described is calcite.
c. Several of the specimens are strongly altered presumably
by hydrothermal solutions. Minerals that are specifically altered
are plagioclase to a fine-grained mineral, perhaps sericite; biotite
to chlorite; and hornblende to what is probably a mixture of finegrained
epidote and chlorite. The K-feldspar is generally unaltered.
d. Apatite is a major constituent of two specimens (HL -1 IB
and HL - 13B).
e. HL10-B contains a fine-grained unidentified yellowish
mineral that forms veinlets and a chalcedony vein let.
f. None of these specimens has a high concentration of
allanite. The mineral marked in HL13-A will be checked by EDX. It
looks like titanite and doesn’t fit the description of allanite in the
report.
g. Specimen HL-13B consists mainly of apatite with small
grains of metamict allanite with radiating fractures.
Then individual thin section analysis was as follows.
i. HL 5A:Partly altered quartzofeldspathic gneiss that consists of quartz, K-feldspar, partly altered (sericitized?) plagioclase, hornblende, and partly chloritized biotite.
ii. HL 5B:Sheared quartzofeldspathic gneiss or granite that consists of quartz, K-feldspar, partly altered plagioclase, hornblende and chlorite.
iii. HL 10A:Quartzofeldspathic gneiss with quartz, K-feldspar, slightly altered plagioclase, partly chloritized biotite (accompanied by titanite), diopside, and rare apatite.
iv. HL 10B:Quartzofeldspathic gneiss that consists of quartz, K-spar, plagioclase, hornblende, and minor titanite. Veinlets of finegrained layer silicate and chalcedony.
v. HL 10C:This specimen consists minimally of K-feldpsar and hornblende with lesser biotite. Biotite is partly chloritized and hornblende is partly altered to epidote. Titanite and apatite are minor constituents.
vi. HL 10D:This cataclasite consists of K-feldspar, altered hornblende, and apatite. A fine-grained layer silicate surrounds K-feldspar and apatite clasts. Allanite was tentatively identified by EDX. Calcite is a trace constituent.
vii. HL 11A:This specimen consists of quartz, K-feldspar, plagioclase, hornblende, and minor apatite. Titanite is a trace constituent.
viii. HL 11B:This cataclasite consists of quartz, K-feldspar, plagioclase, hornblende, and apatite. EDX shows Ba in the K-feldspar indicating that it is hyalophane. Allanite was tentatively identified by EDX. A fine-grained layer silicate surrounds many of the clasts.
ix. HL 13A:This specimen consists of quartz, K-feldspar, diopside, and minor apatite and titanite.
x. HL 13B:This specimen consists mainly of apatite and minor hornblende. The tan, isotropic grains in apatite are tentatively identified to be metamict allanite. Thorite was tentatively identified by EDX. Calcite is a trace constituent.
An example pictures from the mineralogical thin section analysis of sample #1 is illustrated in Figure 1. ICP
Figure 1:Core thin section image example.

ICP Elemental Scans of Core and Composites Samples
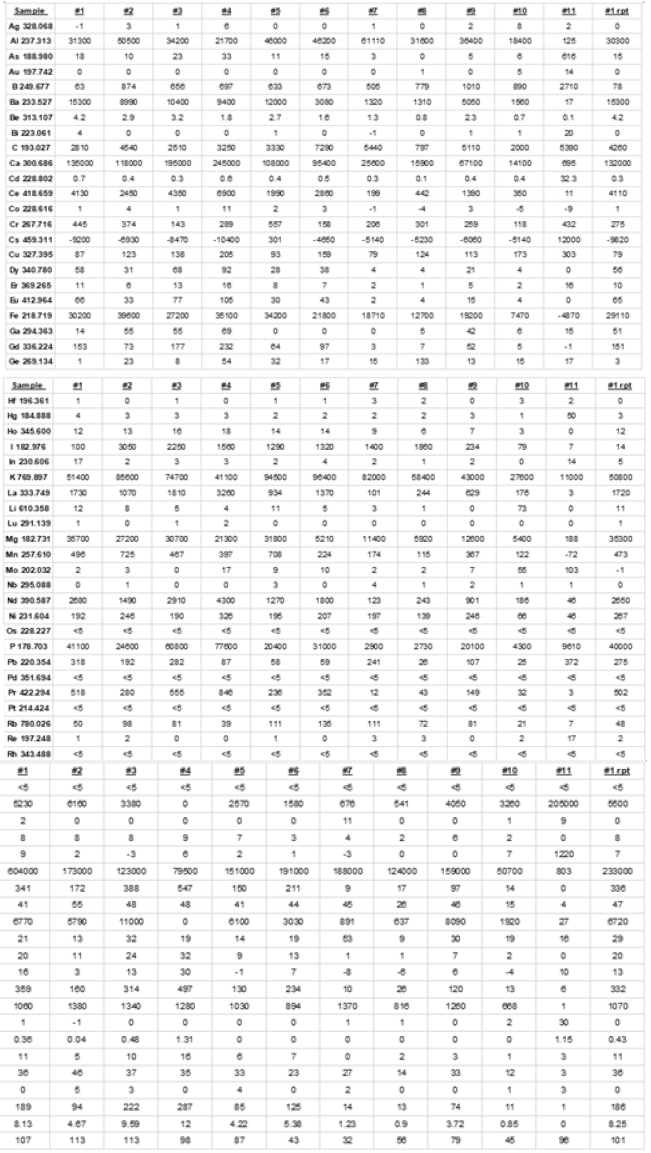
ICP elemental scans were performed for all core samples and the composite and the pertinent analysis for each expressed in ppm follows (Figure 2). Sample #1 HL 10 BX 10 43-2 – 47-4 Sample #2 HL 10 BX 10 39.2-43.2 Sample #3 HL 11 BX 11 44.5-48.7 Sample #4 HL 13 BX 8 34.75 – 39.0 Sample #5 HL 10 BX 12 47.0 – 51.7 Sample #6 HL 5 BX 7 26.7-32.3 Sample #7 HL 10 BX 13 51.7-56 Sample #8 HL 5 BX 8 32.3-36.6 Sample #9 HL 13 BX 9 39.2 – 43.5 Sample #10 HL 11 BX 10 43.3 – 44.5 Sample #11 Composite sample
Figure 2:ICP elemental scans were performed for all core samples and the composite.
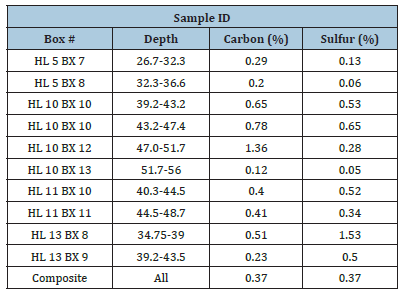
Sample Carbon and Sulfur Analysis
Sulfur and carbon analysis of each core sample and composite was performed. The results are as follows (Table 1).
Table 1:Sulfur and carbon analysis of core sample and composite.
Sem EDX Analysis
Scanning electron microscopy along with energy dispersive x-ray analysis was undertaken on the thin section samples which had been prepared for the thin section analysis. A summary follows. Thin sections, mounted on glass microscope slides, Were commercially prepared. Then examinations, were carried out on the sections with a petrographic microscope [21-30]. A few areas on a few of the slides Were marked as worth examination by SEM/ EDX in order to get some chemical identification of the mineral materials. The SEM/EDX equipment used comprised a LEO 1430VP SEM (operated with a tungsten source), and a PGT Sahara silicon drift detector (LN2-free, resolution about 140ev FWHM at Mn Ka, with detection down to carbon), and the PGT Spirit software suite. The SEM was operated at 20kv with a chamber pressure of 15Pa, so that no coating of the sections was necessary. A quad back scatter detector in the compositional mode was used to image the specimens. This means the contrast in the images was almost entirely due to average atomic number variations.
The general approach was to locate any areas previously marked and to obtain x-ray spectra from various spots within the marked areas. This was mostly done by so-called Spotlight analysis which involves tagging the SEM images at places of interest using the SEM external scan control to then collect automatically an x-ray spectrum from each tagged spot for some period, usually 80 or 100 seconds. The data accompanying this report thus consists of several BSE images showing numbered tagged areas along with the x-ray spectrums from these tagged spots [31-35]. A standardless quantitative analysis was performed on each spot and the result, expressed as elemental and as oxygen compound per cent, is given for each spectrum. It is noted that a carbon peak of some degree was usually present, but carbon was not included in the list of analyzed elements mostly because it was suspected that the carbon came from the cement used to adhere the sections to the glass slides. Aside from the areas marked the sections Were also traversed under the electron beam seeking bright (i.e. metal rich) areas or other features deemed interesting. In the interest of time and expense this search was mostly done at a magnification of lOOX and this means that only a fraction of the total area on a slide was scrutinized in this way. For the most part there were few significant areas containing thorium except for the monazite crystals circled. Rare earth metals were identified as present in all the sections but generally in very small areas. The mineralization is evidently quite complex and zircon crystals were found, occasional pyrite, a strontium mineral, titanium mineral, and barite. The rocks generally were apatite, quartz, and feldspar. We were informed that Scandium at the 2-3gm/t level had been reported in these samples. The encyclopedia states that Scandium is found associated with rare earth elements. This level is essentially below the detectability limit for EDX but even when the electron beam spot is positioned on a rare earth mineral (presumably elevating the Scandium level closer to detectability) the Scandium Ka peak at 4.091kev is located so close to the Calcium KB line at 4.012kev that the overlap renders trace quantities of Scandium invisible. To compound the problem there is a lesser Lanthanum peak even closer to the Scandium peak so that the overlap problem is very formidable [36-41].
X-Ray Diffraction Analysis
X-ray diffraction analysis was also undertaken on the samples. Again, the description, major phases found, and sample numbering was as follows.
Sample #1 HL 10 BX 10 43-2 – 47-4 albite, quartz, fluorapatite, lazulite.
Sample #2 HL 10 BX 10 39.2-43.2 fluorapatite, hydroxylapatite, orthoclase, microline, hyalophane.
Sample #3 HL 11 BX 11 44.5-48.7 fluorapatite, britholite, microperthite, potassium feldspar, orthoclase.
Sample #4 HL 13 BX 8 34.75 – 39.0 microperthite, potassium feldspar, orthoclase, quartz, calcite.
Sample #5 HL 10 BX 12 47.0 – 51.7 quartz, albite, microcline, potassium feldspar.
Sample #6 HL 5 BX 7 26.7-32.3 quartz, potassium feldspar, microcline, albite, fluorapatite.
Sample #7 HL 10 BX 13 51.7-56 fluorapatite, hydroxylapatite, potassium feldspar.
Sample #8 HL 5 BX 8 32.3-36.6 quartz, potassium feldspar, albite, microperthite, fluorapatite.
Sample #9 HL 13 BX 9 39.2 – 43.5 potassium feldspar, orthoclase, microperthit, microcline, albite.
Sample #10 HL 11 BX 10 43.3 – 44.5 quartz, albite, microcline, potassium feldspar.
Sample #11 Composite sample Quartz, albite, microcline.
Bond Grindability of Bulk Blended Composite Sample
The weighted composite sample was tested by conventional Bond work index grindability testing. The work index was found to be 5.72KwHr/ton.
Relative Abrasion Index
Based on the estimated silica content of the composite sample, the relative abrasion index is estimated to be about 0.25lb. of metal per kw-hr of energy used in crushing or grinding.
Summary
Ten boxes of core samples from the Hoidas Lake rare earth deposit were received. Fundamental mineralogical and metallurgical characterizations were carried out on these samples in preparation of beneficiation and hydrometallurgical testing. No mineralogical anomalies surprises were found, and the material appeared representative for subsequent metallurgical testing and process development.
Acknowledgement
Thank you to Dr. Richard Berg of the Montana Bureau of Mines and Geology for his insights and efforts on the SEM EDX research.
References
- Pandur K, Ansdell KM, Kontak DJ, Halpin KM, Creighton S (2016) Petrographic and mineral chemical characteristics of the Hoidas Lake Deposit, Northern Saskatchewan, Canada: Constraints on the origin of a distal magmatic-hydrothermal REE system. Economic Geology 111(3): 667-694.
- U S Department of the Interior, U S Geological Survey, Critical Mineral Resources of the United States-Economic and Environmental Geology and Prospects for Future Supply. In: Schulz KJ, DeYoung JH, Seal RR, Bradley DC (2018) Professional Paper 1802.
- Keller P, Anderson CG (2018) The production of critical materials as by products. Aspects in Mining and Mineral Science 2(2): 208-221.
- Keller P, Anderson CG (2018) Co production of critical energy materials. Research and Development in Materials Science 4(4): 382-383.
- Anderson CG (2016) Industrial manufacture of by product critical materials. Presented at the DOE CMI Annual Meeting.
- Anderson CG (2016) The critical aspect of critical materials. Presented at the DOE CMI Webinar Series.
- Eggert R, Wadia C, Anderson CG, Bauer D, Fields F, et al. (2016) Rare earths: Market disruption, innovation, and global supply chains. Annual Review of Environment and Resources 41: 199-222.
- Imholte D, Nguyen R, Vedantam A, Brown M, Iyer A, et al. (2017) An assessment of U.S. rare earth availability for supporting U.S. wind energy growth targets. Energy Policy 113: 294-305.
- Anderson CD, Taylor P, Anderson CG (2017) Rare earth flotation fundamentals: A review. American Journal of Engineering Research 6(12): 155-166.
- Anderson CD, Taylor P, Anderson CG (2016) Rare earth flotation fundamentals: A review. IMPC 2016 Proceedings of the XXVIII International Mineral Processing Congress.
- Anderson CD (2015) Improved understanding of rare earth surface chemistry and its application to froth flotation. KIEM PhD thesis, Colorado School of Mines, USA.
- Kronholm B, Anderson C, Taylor P (2013) A primer on hydrometallurgical rare earth separations. Journal of Metals 65: 1321-1326.
- Kronholm B, Anderson CG (2020) Upgrade of Yttrium in a mixed rare earth stream using iminodiacetic acid functionalized resin. U S Patent 10,696,562.
- Kronholm B (2019) Upgrade of yttrium in a mixed rare earth stream using iminodiacetic acid functionalized resin. KIEM MSc thesis, Colorado School of Mines, USA.
- Zhang Y, Gu F, Su Z, Liu S, Anderson C, et al. (2020) Hydrometallurgical recovery of rare earth elements from NdFeB permanent magnet scrap: A review. Metals 10(6): 841.
- Schriner D, Anderson C (2015) Metallurgical evaluation of the Hoidas Lake rare earth deposit. Journal of Metallurgical Engineering 4: 69-77.
- Schriner D (2015) Advanced beneficiation of bastnaesite ore through centrifugal concentration and froth flotation. KIEM MSc Thesis, Colorado School of Mines, USA.
- Norgren C (2018) Physical beneficiation of bastnaesite. KIEM MSc Thesis, Colorado School of Mines, USA.
- Everly D (2017) The evaluation of selective collectors for bastnaesite flotation. KIEM MSc Thesis, Colorado School of Mines, USA.
- Everly D, Anderson CG, Jansone-Popova S, Bryantsev V, Moyer B (2020) Flotation of bastnaesite ore using novel collectors. Proceedings of CIM MetSoc Annual Meeting.
- Williams N (2018) Bastnaesite beneficiation by froth flotation and gravity separation. KIEM MSc Thesis, Colorado School of Mines, USA.
- Owens CL, Nash GR, Hadler K, Fitzpatrick RS, Anderson CG, et al. (2018) Zeta potentials of the rare earth element fluorcarbonate minerals focusing on bastnäsite and parasite. Advances in Colloid and Interface Science 256: 152-162.
- Zhang Y, Anderson C (2016) Surface chemistry and microflotation of xenotime and selected gangue minerals using octanohydroxamic acid as the collector. Journal of Sustainable Metallurgy 3: 39-47.
- Zhang Y, Anderson C (2016) A comparison of sodium silicate and ammonium lignosulfonate effects on xenotime and selected gangue mineral microflotation. Minerals Engineering 100: 1-8.
- Zhang Y (2016) Froth flotation of xenotime. KIEM MSc Thesis, Colorado School of Mines, USA.
- Mushidi J, Anderson C (2017) Surface chemistry and flotation behaviors of monazite–apatite–ilmenite–quartz–rutile–zircon with octanohydroxamic acid. Journal of Sustainable Metallurgy 3: 62-72.
- Mushidi J (2016) Surface chemistry and flotation behavior of monazite, apatite, ilmenite, quartz, rutile, and zircon using octanohydroxamic acid collector. KIEM MSc Thesis, Colorado School of Mines, USA.
- Cui H, Anderson C (2016) The simulation and preliminary studies on bear lodge ore flotation. Conference: SME Annual Meeting, At Phoenix, Arizona, Volume: Plant Design Session.
- Cui H, Anderson C (2018) Part of the Minerals, Metals & Materials Series book series (MMMS), Extraction.
- Cui H, Anderson C (2017) Alternative flowsheet for rare earth beneficiation of bear lodge ore. Minerals Engineering 110: 166-178.
- Cui H, Anderson C (2017) Fundamental studies on the surface chemistry of ancylite, calcite, and strontianite. Journal of Sustainable Metallurgy 3: 48-61.
- Cui H (2015) Beneficiation of rare earth element bearing ancylite. KIEM MSc Thesis, Colorado School of Mines, USA.
- Cui H, Anderson C (2020) Beneficiation of ancylite. U S Patent 10,618,058.
- Vaccarezza V, Anderson C (2018) Beneficiation and leaching study of Norra Karr eudialyte mineral. Proceedings of Rare Metals Technology 2018, TMS, 39-51.
- Vaccarezza V (2017) Beneficiation and hydrometallurgical treatment of Norra Karr eudialyte material. KIEM MSc Thesis, Colorado School of Mines, USA.
- Vaccarezza V, Anderson C (2020) An overview of beneficiation and hydrometallurgical techniques on eudialyte group minerals. Mining, Metallurgy and Exploration 37: 39-50.
- Owens CL, Nash GR, Hadler K, Fitzpatrick RS, Anderson CG, et al. (2019) Apatite enrichment by rare earth elements: A review of the effects of surface properties. Advances in Colloid and Interface Science 265: 14-28.
- Anderson CG, Miranda PJ (2020) Metallurgical evaluation of the Hoidas Lake rare earth deposit. Metallurgical and Materials Engineering 26(2): 143-162.
- Norgren C, Anderson C (2021) Ultra-fine centrifugal concentration of bastnaesite ore. Metals 11(10).
- Norgren C, Anderson C (2022) Recovery of rare earth oxides from flotation concentrates of bastnaesite ore by ultra-fine centrifugal concentration. Metals.
- Colligan G, O Kelley B, Anderson C (2022) Novel methods for bastnaesite concentrate leaching. Mining Metallurgy & Exploration Journal 39: 31-38.
© 2022 Corby G Anderson. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






