- Submissions

Full Text
Aspects in Mining & Mineral Science
Catalytic Method of Corrosion on the Surface by Dispersed Inclusions
Bragin VI1,2 and Mikhailov AG2*
1Siberian Federal University, Russia
2Institute of Chemistry and Chemical Technology SB RAS, Russia
*Corresponding author: Mikhailov AG, Institute of Chemistry and Chemical Technology SB RAS, Akademgorodok, 50, building 24, Krasnoyarsk, Russia
Submission: September 06, 2022;Published: September 19, 2022

ISSN 2578-0255Volume9 Issue5
Abstract
The pitting mechanism of catalytic destruction (weathering) of the surface has been proposed and experimentally confirmed. The processes of weathering (oxidation) with the destruction of the structure and a change in the material composition in parts of the subsoil, in which platinum is involved, occurs at an accelerated rate. Experimental results of pitting corrosion of metal and sulfide surfaces are presented.
Keywords:Pitting; Surface; Sulfide; Corrosion; Dispersed particles
Introduction
The integrity, strength and durability of the material largely depend on the resistance to aggressive environments. Material transformations in the zone of hypergenesis are caused by various factors. The destruction of mineral structures is partly due to the action of climatic fluctuations in FT [1]. The physical destruction of crystals and their aggregates significantly increases the contact area of the available surface of the massif particles, which activates chemical [2] and biochemical [3] transformations of the massif. An increase in the porosity of the massif promotes the filtration mobility of fluids. The most intense transition to the soluble form of natural minerals, including sulfides [4-6] is observed precisely under conditions of hypergenesis. Intensive infiltration processes in the hypergenesis zone led to the formation of deposits of a number of minerals. Filtration mass transfer serves as a transport mechanism for the real transformation of the array. This principle underlies real technological solutions for in situ leaching of metallic ores [7] and non-metallic ores such as Chilean nitrate [8]. Destruction of minerals, conversion into soluble form and subsequent dissolution precedes filtration mass [4]. Destruction of minerals, conversion into a soluble form and subsequent dissolution precedes filtration mass transfer [9]. The initial process of destruction of the surface of minerals is important and largely determines the direction of transformation of the real transformation [10]. Initial The stage of transformation of sulfide minerals has its own specifics. Experimental studies were carried out to substantiate the assumption about the pitting mechanism of sulfide corrosion.
Proposed Mechanism for Sulfide Transformation
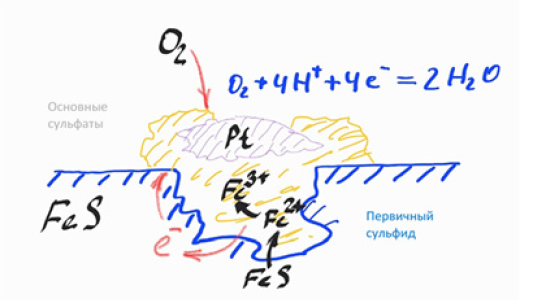
The main process regulating the weathering of sulfide-containing materials is the oxidation of sulfides with the formation of hydrated iron oxides and free sulfuric acid [11]. Almost all other changes are associated with this reaction. Previous studies have shown that the weathering of sulfides containing dispersed platinum has a higher rate of release of dispersed platinum particles from the sulfide matrix compared to the rate of oxidation of sulfides. As a result, there is a sharp decrease in the proportion of platinum in granular classes and its concentration in slimes. It is essential that platinum is redistributed into the sludge even before the complete oxidation of the carrier minerals – non-ferrous metal and iron sulfides. It has been experimentally established that accelerated corrosion follows the pitting mechanism.It is typical for local inhomogeneities associated with dispersed inclusions, which have very different electrochemical properties, and have a sharp acceleration of the process in the presence of even small amounts of halide ions, primarily chlorine ions. The previously obtained experimental data with dispersed platinum on the surface fully correspond to the proposed mechanism (Figure 1): Platinum is present in the form of dispersed metallic inclusions, which create inhomogeneities in the volume and on the surface of minerals, on which pitting can develop: Platinum and palladium and have catalytic properties and increased adsorption capacity for oxygen. The hydrogen overvoltage on their surface is close to zero, in contrast to the surface of the sulfide matrix, and therefore the dispersed inclusions of platinum and palladium are the centers of sulfide corrosion. It should be noted that in addition to acceleration of corrosion near contact with platinum, sulfide oxidation also occurs along other inhomogeneities, in particular, near inclusions of sulfides of a different composition, rock-forming minerals, and silver (Figures 2 & 3). The mechanism of accelerated weathering in the presence of dispersed platinum inclusions is shown in the Figure 4. The main factor of the mechanism is the oxygen depolarization of the cathode. Inclusions that catalyze the depolarization reaction increase the cathode current and thereby accelerate the process. The role of chloride ions, as in the case of conventional pitting, is to increase the solubility of iron oxide near the anode.
Figure 1:Platinum nanoparticles deposited on the surface of iron (a) and the appearance of the surface after 2 weeks in a humid atmosphere (b).
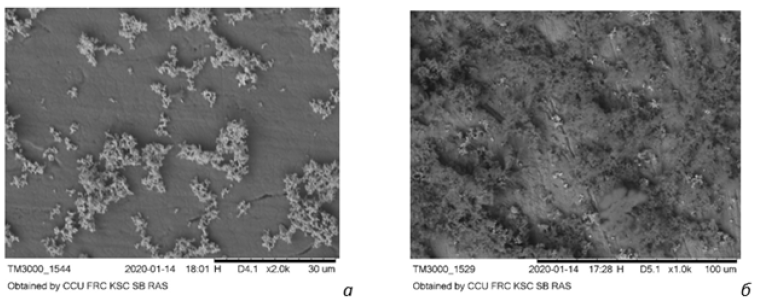
Figure 2:Oxidation center formed by an inclusion in pyrite.
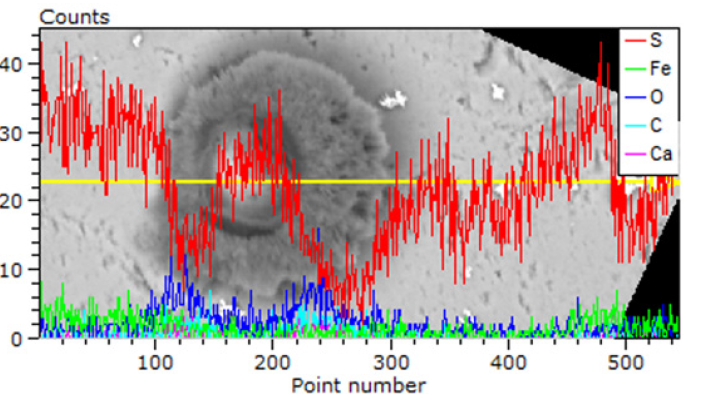
Figure 3: Corrosion at the contact of pyrrhotite particles in the host rock.
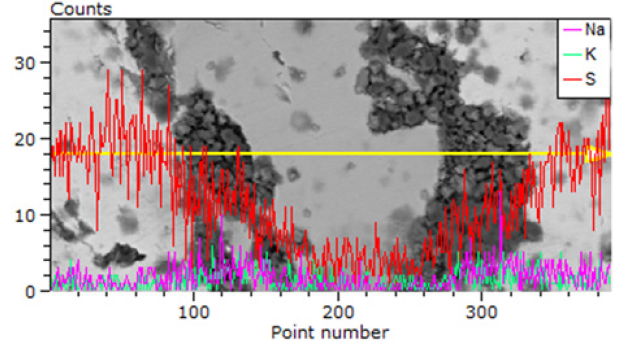
Figure 4: The mechanism of corrosion of the surface of sulfides during catalytic acceleration by dispersed inclusions.
Conclusion
The factors controlling the accelerated weathering of the surface of metals and sulfides are the quality of the electrical contact of the metal particle with the surface, the presence of moisture and halide ions. The accelerated destruction of the surface is determined by the catalytic action of the metal under these conditions, which leads to depolarization of the anode and an increase in the cathode current. With the further development of the corrosion process, continuous Fe oxide films are formed on the surface of metals and sulfides, including dispersed particles of platinum. Thus, as a result of research, the acceleration of destruction (weathering) on the surface of metals and sulfides near inclusions and in the zone of contact with dispersed platinum particles was experimentally shown. A mechanism for accelerating destruction from the surface (weathering) has been proposed and experimentally confirmed. The phenomenon of accelerated weathering of sulfides and the concentration of platinum in the sludge products of weathering is explained by the studies carried out.
References
- Matsuoka N, Murton J (2008) Frost weathering: recent advances and future directions/Special Issue: Recent advances in permafrost and periglacial research. Ninth International Conference on Permafrost, Fairbanks, Alaska, USA 19(2): 195-210.
- Koponen HT, Martikainen PJ (2004) Soil water content and freezing temperature affect freeze–thaw related N2O production in organic soil. Nutr Cycl Agroecosyst 69: 213-219.
- Feng X, Nielsen LL, Simpson MJ (2007) Responses of soil organic matter and microorganisms to freeze–thaw cycles. Soil Biol Biochem 39(8): 2027-2037.
- Lottermoser BG (2010) Mine wastes, characterization, treatment and environmental impacts. Springer: Berlin/Heidelberg, Germany, p. 400.
- Heikkinen PM, Räisänen ML, Johnson RH (2009) Geochemical characterisation of seepage and drainage water quality from two sulphide mine tailings impoundments: Acid mine drainage versus neutral mine drainage. Mine Water Environ 28: 30-49.
- Krasavtseva EA, Makarov DV, Selivanova EA, Maksimova VV, Svetlov AV (2021) Mobilization of ecologically hazardous elements from the tailings of loparite ore concentration under the influence of atmospheric precipitation. Geo-ecology Eng Geol Hydrogeol Geocryol 3: 69-78.
- Dixon DG (2003) Heap leach modeling-the current state of the art. Hydrometallurgy-Fifth International Conference, pp. 289-314.
- Moncur MC, Ptacek CJ, Hayashi M, Blowes DW, Birks SJ (2014) Seasonal cycling and mass-loading of dissolved metals and sulfate discharging from an abandoned mine site in northern Canada. Appl Geochem 41: 176-188.
- Steiger M (2005) Crystal growth in porous materials-II: Influence of crystal size on the crystallization pressure. J Cryst Growth 282(3-4): 470-481.
- Mazukhina SI, Masloboev VA, Makarov DV (2021) Thermodynamic modeling of hypergene processes in copper-nickel ore concentration tailings when exposed to different temperatures and moisture levels. Chem Sustain Dev 29: 69-79.
- Chekushin VS, Oleinikova NV, Baksheev SP, Dontsov AV (2020) Direct reduction of heavy non-ferrous metals from sulfide compounds. Non-Ferrous metallurgy 5: 4-12.
© 2022 Mikhailov AG. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






