- Submissions

Full Text
Aspects in Mining & Mineral Science
Physical and Chemical Characteristics of Microsilica Formed During Ferrosilicon Smelting
Akhmadjonov AA and Kadyrova ZR*
Institute of General and Inorganic Chemistry of the Academy of Science of the Republic of Uzbekistan77a Mirzo-Ulugbek st, Uzbekist
*Corresponding author: Kadyrova ZR, Institute of General and Inorganic Chemistry of the Academy of Science of the Republic of Uzbekistan77a Mirzo-Ulugbek st, 100170, Tashkent, Uzbekistan
Submission: August 05, 2022;Published: August 23, 2022

ISSN 2578-0255Volume9 Issue5
Abstract
The results of a study of the physicochemical characteristics of microsilica formed during the smelting of ferrosilicon produced by Uzmetkombinat JSC are presented to establish the possibility of their integrated use in a number of industries, in particular, first of all, in the construction industry, in the rubber industry, in the production of refractories and silicate materials and in metallurgy. The technological feasibility of using microsilica in various industries of the Republic of Uzbekistan has been established.
Keywords:Microsilica; Ferrosilicon; Smelting; Waste; Metallurgical dust; Chemical; Mineralogical; X-Ray phase; Electron microscopic; Magnetic separation
Introduction
It is known [1,2] that large resources of dusty wastes of silicon and high-silicon ferroalloys production, containing significant amounts of silicon dioxide, predetermines the relevance of the problem in a case of their rational use. However, at present, most enterprises continue to accumulate microsilica. This in turn of leads to economic losses associated, on the one hand, with the non-use of industrially valuable waste and, on the other hand, with the costs of its storage. In addition, as a result of indiscriminate collection and accumulation dust wastes often lose their value as raw materials for possible processing, and the current methods of storage of Microsilica (MSc) are not environmentally friendly. Therefore, utilization and usage of dust emissions should be considered as an important direction of saving material resources, as well as increasing the efficiency of environmental protection in the production of siliceous ferroalloys. The annual production of microsilica formed in the process of smelting ferrosilicon on the example of JSC Uzbek Metallurgical Plant is 4500 tons/year. The formation of such waste, together with other metallurgical waste, leads to a deterioration in the environmental situation of the enterprise and also the nearest region. In this regard, within the framework of the Green Chemistry Program, we carried out a physical and chemical study on the utilization of microsilica in order to use it as a raw material component in the production of silicate materials for various purposes.
The analysis of published works revealed that silicon oxide (silica) is contained in a large number of multi-tonnage industrial wastes, in particular in mining wastes contains in ranges 20-80% SiO2 [3,4], in metallurgical wastes ranges 16-55% [5], in ash and ash slag of thermal units is 27-63% [6]. Its highest content is found in the wastes of ferroalloys production which makes up to 92% [7]. Dusty wastes of ferroalloys production are condensed as aerosols and according to the classification belong to the category of fumes. To date, the following terminology, uniting this type of waste “silica powder”, “microsilica”, “silica fume”. It should be noted that in domestic practice the term “microsilica fume” is usually used [8]. Microsilica is used as a raw material for the production of silicate materials, in particular, in the construction industry to replace silicate materials for the production of glass products, materials and increase the workability of strength, reduce the setting time of the concrete mix [9], in the production of dinas refractories to replace natural quartzite increase strength of acidic refractory products [10]. In the rubber industry in reducing environments, it is used as a sealing agent to reduce tread wear, increase tire life, reduce rolling resistance and enhance frictional properties [11,12]. In granular form with coarsely dispersed raw materials as a secondary resource, it is returned in the process of obtaining ferrosilicon, while a method has been developed for agglomerating silica fume from silicon production with liquid glass [13,14]. In general, such a study of the use of microsilica allows to reduce the consumption of charge materials and electricity in the above industries.
Thus, a significant number of works by native and foreign authors have been devoted to the study of the properties of microsilica produced by ferrosilicon. The physical and technological characteristics of microsilica have been most fully investigated in order to study the possibility of its use in the construction industry, the most traditional field [15,16]. The physicochemical evaluation of microsilica for ferroalloys production is also usually of an applied nature and is performed mainly according to its chemical composition and dispersibility. In the production of ferroalloys silicon oxide, in the form of microsilica is accompanied by the formation of large amounts of dusty wastes, which are released with gaseous products. In this case, in the example of JSC “Uzmetkombinat” specific number of emissions in the form of microsilica dust formed during production of ferrosilicon grades FS45, is 0.465g/tn. The content of silica in dusty wastes usually increases with increasing of silicon content in alloy [17,18] and amounts ~60% in melting of low-percent ferrosilicon grades (FS18- FS45) and up to 90% - in melting of high-percent (FS65-FS75). The process of formation of microsilica occurs due to mechanical entrainment of small fractions of the charge of ferrosilicon production. The formation of microsilica as a result of oxidation reactions in the gas ducts occurring mainly in oxidation furnaces can be described in the following way:

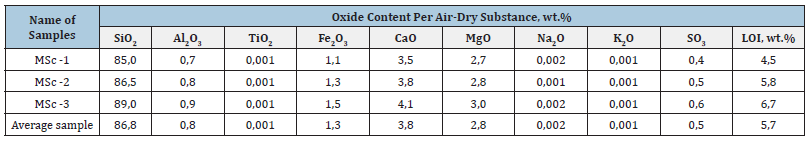
The chemical composition of microsilica sample formed during the production of ferrosilicon by JSC “Uzmetkombinat” depends on the grades of alloys being smelted and was determined by standard methods of silicate technology [19,20]. It was found that the content of the main phase - silica in ferrosilicon wastes is 85.0- 89.0wt%. Free carbon in microsilica is 0.5-1.2wt.%, free silicon is 0.12-0.18wt.%. Losses during calcination of microsilica at 1000 °C amount to 4.5-6.7wt.%, which agrees with the results of differential thermal analysis. The chemical composition of microsilica samples also includes free chemical elements in the form of carbon and silicon, the remaining elements are mainly in the oxide form. The results of the study of the chemical composition of microsilica are shown in Table 1. The formed microsilica is a finely dispersed powder of gray color. The samples of microsilica of ferrosilicon gas purification are characterized by low bulk density, which is 0.25-0.32g/cm3. Fractional composition of the investigated samples is within 0.0006-0.0010mm, depending on the alloy grade. The moisture content of microsilica depending on storage and atmosphere is in the range of 1.5-3.0%. In [8] it was found that the adsorption capacity of the microsilica sample is relatively low, hence the amount of water required to create one molecular layer of microsilica is 6.336.10-3g/m2. In this case the number of monolayers of water adsorbed on particles is 3-4. At the same time this amount of water is sufficient for the formation of a gel-like phase on the surface of microsilica particles, which increases the adhesion. Hydraulic activity of microsilica estimated by the amount of lime absorbed from the saturated solution at 85 °С and water consumption are 100-104kg СаО/t and 40-42%.
Table 1:Results of the chemical analysis of samples Microsilica of JSC “Uzmetcombinat”. The index of Loss on Ignition (LOI) includes water, organic and volatile impurities and carbon dioxide (CO2).
Methods
To determine the crystalline phase of the experimental samples of microsilica used X-ray phase analysis. X-ray phase analysis of sample samples was carried out by the powder method on an X-ray diffractometer brand LABX XRD-6100 SHIMADZU in the range of 2θ, 10-80 using CuKα radiation with a wavelength of 1.5418 Å. X-ray photographs were taken with a step of 0.02 deg, voltage 30kV, current 30mA. International standard reference data [21,22] were used in calculations and in the identification of crystalline phases. As a result of the identification of XRD patterns of the initial microsilica it was found that the phase composition of microsilica is XRD amorphous. Therefore, the X-ray diffraction patterns of microsilica sample cause difficulties in phase identification, which is also noted in [3,8]. In this regard, the magnetic fraction was demagnetized in order to determine the crystalline components in the mass of microsilica.
Results and Discussion
Based on the results of the identification of X-ray diffraction phases, it was found that the obtained magnetic fraction of microsilica consists of crystalline silicon, magnetite, and hematite. The XRD pattern also revealed insignificant diffraction maxima related to α-quartz, α- and β-silicon carbide minerals (Table 2). Silicates such as α-CaSiO3, Al2O3·SiO2, Ca(Mg,Fe)3(SiO3)4 and silicon carbide were identified in the non-magnetic fraction, while only silicon carbide was identified in the heavy fraction containing a small amount of crystalline phase. The study of the structural characteristics, in particular the shape and size of microsilica particles, was carried out by electron microscopic analysis. The images were obtained using a Carl Zeiss SEM MA 10 scanning electron microscope using Signal A=SE 1 backscattered electrodes under the following imaging conditions: ENT voltage - 15.0kV, working distance WD - 8.5mm. Description of crystalline phases and deciphering of structural characteristics of experimental samples were carried out with the help of the reference book [23,24]. The preparations were prepared as follows. Microsilica powder was sprayed on a 10x8x1mm pre-rolled indium plate, the sawn layer was pressed in, and the residue was removed by blowing on the surface. Next, particles impregnated in a metal matrix were investigated. The powders were magnified up to 300000. The study of microphotographs obtained by Scanning Electron Microscopy (SEM) shows that the species of microsilica in the state of supply are represented by spherical aggregates of different sizes, varying in a wide range from 100 to 600nm. Individual aggregates are formed by spherical particles mostly 50-80nm in size, the number of which in the aggregate depends on its coarseness (Figure 1).
Table 2:XRD characteristics of the magnetic fraction of silica fume produced by ferrosilicon JSC “Uzmetkombinat”.
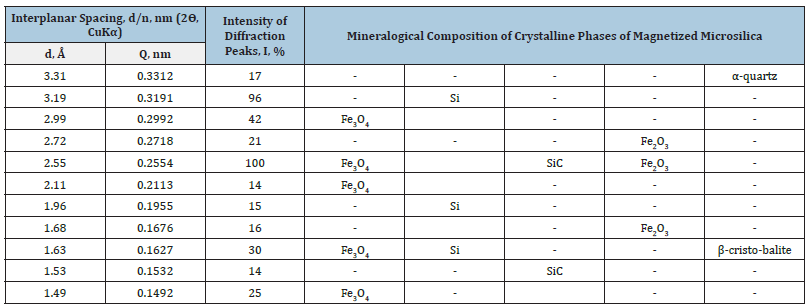
Figure 1: Electron microscopic images of microsilica.
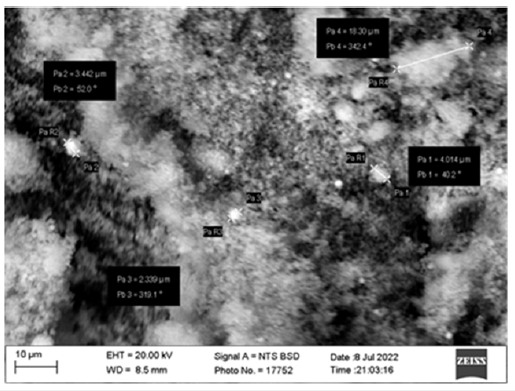
The results obtained by SEM methods make it possible to trace the morphological structure of aggregates, shape and size of individual particles of microsilica. In general, it shows that microsilica formed during ferrosilicon production consists of particles mostly of spherical shape sized 0.1-0.4μm, combined into aggregates sized up to 1μm. In some areas of the spectrum the increase in the size of particles is due, as a result of the preparation of samples - preparations for electron microscopic studies.
Conclusion
Thus, the main characteristics of microsilica formed during the melting of ferrosilicon produced by JSC “Uzmetcombinat” have been studied to establish the possibility of their integrated use in a number of industries, in particular, primarily in the construction industry, in the rubber industry, in the production of refractory and silicate materials, in metallurgy. The results obtained confirm the technological expediency of microsilica application in various industries of the Republic of Uzbekistan.
References
- Vinogradov SV (1989) Prospects of using the gas-cleaning dust of ferrosilicon production. Stal 4: 41-44.
- Maksimov YS (1978) Dust-like wastes during melting ferrosilicon Production of ferroalloys. Metallurgy pp. 81-84.
- Polyakh OA (2005) Analysis of formation conditions and physicochemical attestation of microsilica. In: Polyakh OA, Galevsky GV, Yakushevich NF (Eds.), Waste management - the basis of restoration of ecological equilibrium in Kuzbass. First International Scientific and Practical Conference, SibGIU, Novokuznetsk, Russia, pp. 224-229.
- Bazhenov PI (1986) Building ceramics from industrial by-products. Moscow, Russia, p. 136.
- Ryabov GG (1988) Research of metallurgical slags for making putties. Review information. VNIIESM. Ser.11: Utilization of by-products waste in building materials and products manufacture (in Russian), pp. 9-11.
- (1985) Composition and properties of ash and slag from TPPs: Reference. PART I. - Moscow: Energoatomizdat, Russia, p. 288.
- Kaprielov SS (1992) Effective way of utilization of ultradispersed products of furnace gas cleaning. Steel 5: 83-85.
- Kolosov AD, Nemarov AA, Nebogin SA (2017) Technology for the production and use of nanosilica in the production of new materials for mechanical engineering Modern technologies. System Analysis, Modeling 3(55): 59-66.
- Batrakov VG (1990) Estimation of ultra-dispersed wastes of metallurgical productions as additives in concrete. Concrete and Reinforced Concrete 12: 15-17.
- Snitko YP (1997) Utilization of dry dust from the production of ferrosilicon. Improving the production of ferrosilicon: materials of the factory scientific and technical. Conf 3: 349-360.
- Babaev ZK, Ibragimov DU, Karimov SH, Kenzhaev FD, Yadgorov AM (2018) State and development of the glass industry of Uzbekistan. Chemical Technology 2(47): 1503.
- Goncharov YI (2009) Raw materials silicate industry. Publishing house of the Association of Building Universities, Moscow, Russia, p. 124.
- Akhmedov NA, Isakhodzhaev BA, Popov EL (2006) Technogenic wastes of enterprises of Uzbekistan and prospects for their processing. Mining Bulletin of Uzbekistan 1(24): 19-23.
- Vertiy IG (1987) Utilization of pulverized and slag waste from the production of silicon ferroalloys. Steel 8: 42-43.
- Evseev NV, Tyutrin AA, Pastukhov MP (2019) Granulation of dust waste from silicon production for return to the technological process. Bulletin of the Irkutsk State Technical University, Russia, 23(4): 805-815.
- Gasik MI, Lyakishev NP (1999) Theory and technology of electrometallurgy of ferroalloys. Internet Engineering p. 764.
- Kaprielov SS (1992) Effective way of utilization of ultradispersed products of gas cleaning of furnaces. Steel 5: 83-85.
- Storozhenko GI, Cherepanov KA (1989) Determination of the main characteristics of the pulverized waste of ferrosilicon production. Ferrous Metallurgy 2: 152-155.
- Vakalova TV, Habas TA, Reva IB (2013) Workshop on the basics of technology of refractory nonmetallic and silicate materials. Izd Tomsk PU, p. 176.
- Dyatlova EM, Biryuk VA (2006) Chemical technology of ceramics and refractories. Laboratory practical work. Minsk, BSTU, p. 284.
- Tolkachev SS (1968) Tables of interplanar distances. Chemistry, p. 100.
- (2005) ASTM Standards Part 17, Refractories, Glass, Ceramic Materials, Carbon and Graphite Products. ASTM, Philadelphia, USA, pp. 7-9.
- (2021) Raster electron microscopy for nanotechnology. Methods and applications. In: Zhu W, Wang JL (Eds.), (4th) Electronic. Laboratory of Knowledge, p. 601.
- Vlasov AI, Elsukov KA, Kosolapov IA (2011) Electron microscopy. Publishing house of the Bauman Moscow State Technical University, Russia.
© 2022 Kadyrova ZR. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






