- Submissions

Full Text
Aspects in Mining & Mineral Science
CaO-Al2O3 System and Possibilities of Sol-Gel Synthesis
Khomidov FG, Kadyrova ZR*, Usmanov KL and Niyazova SM
Institute of General and Inorganic Chemistry of the Academy of Science of the Republic of Uzbekistan, Uzbekistan
*Corresponding author: Kadyrova ZR, Institute of General and Inorganic Chemistry of the Academy of Science of the Republic of Uzbekistan, Uzbekistana
Submission: August 05, 2022;Published: August 19, 2022

ISSN 2578-0255Volume9 Issue4
Abstract
Compounds of calcium monoaluminate were synthesized using the sol-gel method. The effect of soluble aluminum and calcium salts on the synthesis, kinetics, and mechanism of the phase formation reaction during sintering of calcium monoaluminate in the temperature range of 500-1000 °C has been studied. The formation of calcium monoaluminate occurs through an intermediate compound - the mineral maenite.
Keywords:Calcium aluminate; Oxide compounds; Sol-gel method; Dispersed system; Eutectic; Melting point
Introduction
Figure 1:State diagram of the CaO system-Al2O3 built based on the latest data [5].
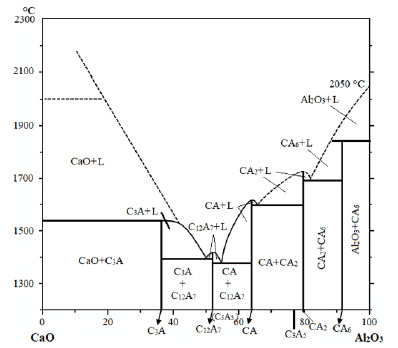
Calcium aluminates - are among the most widely studied compounds that are part of a number of technical products, such as aluminous cement, portland cement, some special cements, abrasive products, phosphors, etc. Also, they are widely used in ceramics, as a binder in refractory cast products, for the steel industry of detectors, biomaterials and optical devices. They have different crystal structures and are formed during the production of a number of chemical products. They are not found among natural materials, however, as intermediate compounds, they can be formed during the formation of rock eruption [1,2]. Many works [3,4] are devoted to research on the study of the physico-chemical properties of calcium aluminates. The following five individual chemical compounds are mainly formed in the CaO-Al2O3 system: 3CaO‧Al2O3, 5CaO‧3Al2O3 (12CaO⸳7Al2O3), CaO‧Al2O3, CaO‧2Al2O3, CaO‧6Al2O3. Calcium aluminate CaO‧Al2O3 is one of the most widely studied oxide systems. This is mainly due to the hydraulic properties of some oxide compounds in this system - CaO‧Al2O3 (CA), CaO‧2Al2O3 (CA2), 12CaO‧7Al2O33 (C12A7) - used during the production of aluminous cement. Two other compounds - CaO·6Al2O3 (CA6) and 3CaO·Al2O3 (C3A) - are not included in the composition of high alumina cement. For the first time, Rankin and Wright investigated and built a state diagram of the CaO‧Al2O3 compound system (Figure 1) [5]. Subsequently, the composition of some compounds was refined and a new compound CaO‧6Al2O3 was discovered. Also, some authors believe that the 3CaO‧5Al2O3 compound described in Rankin's works actually corresponds to the formula CaO‧2Al2O3, which was determined by Tawashi, and this formula is generally accepted.
Based on this state diagram, the invariant points of the compounds of the CaO‧Al2O3 system were determined (Table 1). Chemically prepared powders are considered to be the most common type of starting material in the manufacture of ceramics. During the manufacturing process, materials are powdered to obtain ceramic particles of the desired size. Unfortunately, the processing of such powders remains a problem, although the resulting properties, such as high mechanical, electrical and thermal properties of coarse granular ceramics, differ significantly from their traditional homologues. The main steps in the manufacture of nanoceramics are associated with obtaining non-agglomerated nanopowders with a uniform size distribution and sintering to a theoretical density without grain growth. Chemical methods for obtaining the synthesis of ceramic powders can basically be divided into three classifications, depending on the medium in which the physical and chemical process occurs - liquid, gas, plasma [6-10]. Synthesis in a liquid medium: precipitation methods, heterophase synthesis, solgel method, hydrothermal method; Synthesis in a gaseous medium: gas-phase synthesis, interaction of a solid body with a gas and processes of decomposition of salts, hydroxides, organoelement compounds; Synthesis with the participation of plasma: Plasmachemical, electroerosive, self-propagating high-temperature synthesis. Reducing the temperature of synthesis and sintering in the technology of obtaining ceramic materials remains an urgent task, the solution of which leads to savings in fuel and energy resources. Recently, many scientists have paid special attention to the synthesis of the above materials using the sol-gel method [11]. In industry, more than 70-80% of MgAl2O4 spinel is produced by the solid-phase reaction method. The synthesis of spinel requires homogeneous, highly reactive and non-agglomerated powders of starting components with a firing temperature of more than 1600 °C, which is necessary to complete spinelization through solid-state reactions.
Table 1:Invariant points of the CaO‧Al2O3 system [5].
The sol-gel method has a number of advantages, such as: to form the necessary phase compositions and structure of the material at lower temperatures (several hundred degrees) than those of traditional technologies, the possibility of obtaining powders with a controlled particle size distribution; the possibility of obtaining high purity and dispersion (100-10nm); as well as high homogeneity of the material [12]. The sol-gel method of obtaining glass and ceramics from metal oxides is carried out by chemical hydrolysis to form a sol, and then a gel, which, when dried and pyrolyzed, produces an amorphous oxide [13]. In this work, CaAl2O4 was synthesized by the sol-gel method. The sol-gel method makes it possible to form the necessary phase compositions and material structure at lower temperatures.
Experiment
For the study, 4-aqueous calcium nitrate (Ca(NO3) 4H2O grade 99.9) and 9-aqueous aluminum nitrate (Al(NO3)3‧9H2O grade 99.9) as well as citric acid were used as initial components. The phase composition of the materials used and the synthesized calcium aluminate powder was determined on a LABX XRD-6100 SHIMADZU diffractograph using CuKα radiation and a Ni filter with a wavelength of 1.5418 Å. 4-aqueous calcium nitrate (Ca(NO3) 4H2O) and 9-aqueous aluminum nitrate (Al(NO3)3‧9H2O) were dissolved in distilled water at room temperature. After stirring, citric acid was added to the resulting solution. The precursor solution was stirred on a magnetic stirrer at a temperature of 70 °С until a gel-like mass was obtained. The resulting gel-like mass was dried at a temperature of 130 °C in an oven to obtain a xerogel. To determine the formation of the crystal structure, monocalcium aluminate and the influence of the exposure time during heat treatment on the synthesis process and the complete completion of the phase formation of tricalcium aluminate, the dried gel was fired at a temperature range from 500 to 1000 °C with an exposure of 120 minutes in a SNOL 5/1300 muffle furnace.
Results and Discussion
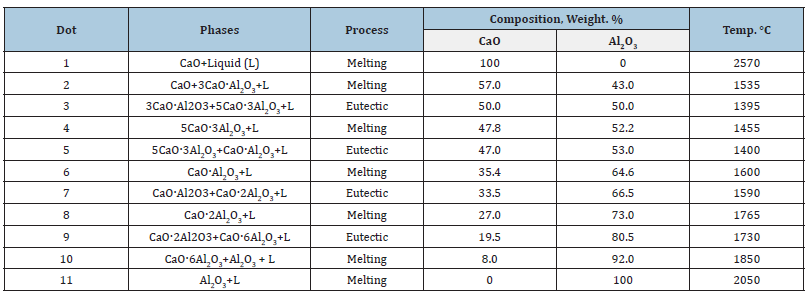
The results of X-ray phase analysis of fired samples in the temperature range of 500-1000 ℃ are shown in Figure 2. On the X-ray diffraction patterns of the synthesized samples at a temperature of 500 °C, the beginning of the formation of calcium monoaluminate (d=0.467, 0.297, 0.251, 0.192nm) and the intermediate compound maenite (0.244, 0.152nm) was observed. With an increase in temperature to 800 °C, an intensive increase in the diffraction lines of calcium monoaluminate, as well as the mineral maenite, was observed. However, with an increase in temperature to 1000 °C, an intensive formation of calcium monoaluminate occurs, due to a decrease in the amount of maenite, which precedes the formation of calcium monoaluminate.In this temperature range, no diffraction lines are observed corresponding to calcium and aluminum oxides, which are in an amorphous state as a result of the decomposition of the corresponding nitrate compounds. Hence aluminum nitrate decomposes to γ-Al2O3 while Ca(NO3)2 decomposes in part to form calcium oxide. The resulting CaO reacts intensely with Al2O3, forming maenite Ca12Al14O33 (C12A7). It should be noted that the formation of C12A7 via the CaOAl2O3 reaction is exothermic and has a high negative ΔG (energy). When introducing more energy into the system through increasing temperature, Ca(NO3)2 decomposes in large quantities and melts them, forming an amorphous form of CaO. In this temperature range, no diffraction lines are observed corresponding to calcium and aluminum oxides, which are in an amorphous state as a result of the decomposition of the corresponding nitrate compounds. Hence aluminum nitrate decomposes to γ-Al2O3 while Ca(NO3)2 decomposes in part to form calcium oxide. The resulting CaO reacts intensely with Al2O3, forming maenite Ca12Al14O33 (C12A7).
Figure 2: X-ray diffraction patterns of the synthesized samples in the temperature range 500-1000 ℃.
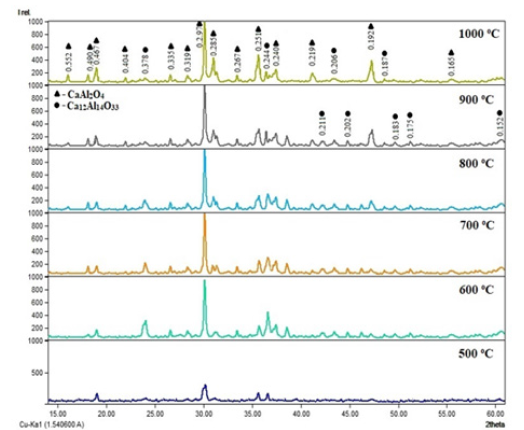
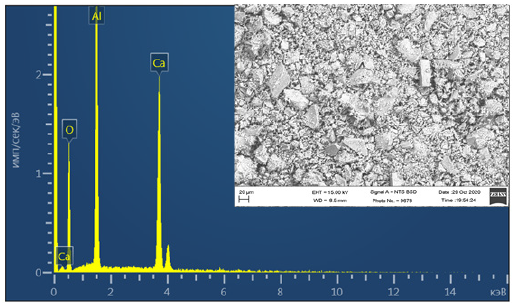
When introducing more energy into the system through increasing temperature, Ca(NO3)2 decomposes in large quantities and melts them, forming an amorphous form of CaO. Transition phases begin to crystallize, as Ca12Al14O33 passes to CaAl2O4 with an increase in the amount of energy. Figure 3 shows the kinetic parameters of CaAl2O4 phase formation at a sintering temperature of 1000 ℃ using precursors. It is known that in solid-phase synthesis, a temperature above 1500 ℃ is required for the complete formation of calcium monoaluminate. Consequently, during solid-phase synthesis in the composition γ-Al2O3+CaCO3 at a temperature of 1000 ℃, crystalline phases of calcium monoaluminate are formed in a smaller amount, compared with the synthesis of samples by the sol-gel method. The obtained results of SEM and EMF analyzes of the synthesized calcium monoaluminate by the sol-gel method are shown in Figure 4. The obtained results of SEM analysis show that the calcium monoaluminate synthesized by the sol-gel method also has a nanoporous structure, in which clearly formed particles of the rhombic structure of calcium monoaluminate are found. The presented EMF spectra confirm that in each sample of calcium monoaluminate, only the chemical elements Ca, Al and O are found on the surface
Figure 3: Kinetic parameters of sintering at 1000 ℃ of CaAl2O4 phase formation using precursors: 1 – Al(NO3)3+Са(NO3)2; 2 – AlСl3+Са(NO3)2; 3 – Al(OH)3+Са(NO3)2; 4 – γ-Al2O3 CaCO.
Figure 4: SEM and EMF images of CaAl2O4 synthesized at 1000 °C.
Conclusion
Thus, calcium monoaluminate compounds were synthesized using the sol-gel method. The effect of soluble aluminum and calcium salts on the synthesis, kinetics, and mechanism of the phase formation reaction during sintering of calcium monoaluminate in the temperature range of 500-1000 °C was studied. It has been established that the formation of calcium monoaluminate occurs through an intermediate compound - the mineral maenite. When the temperature rises to 1000 °C, maenite precedes the formation of calcium monoaluminate.
References
- Mercury Rivas JM, De Aza AH, Pena P (2005) Synthesis of CaAl2O4 from powders: Particle size effect. Journal of the European Ceramic Society 25(14): 3269-3279.
- Ianoş R, Lazău I, Păcurariu C, Barvinschi P (2009) Fuel mixture approach for solution combustion synthesis of Ca3Al2O6 Cement and Concrete Research 39(7): 566-572.
- Maria CD, Gilberto A, Masciocchi N, Giacobbe C, Castiglioni F, et al. (2021) The crystal structure of a new calcium aluminate phase containing formate. Cement and Concrete Research 146: 106490.
- Wang B, Liu J, Sun H, Zhang Y, Liu D (2015) Synergistic effect of C12A7 and CA on alumina leaching property under low calcium/aluminum ratio. Light Metals 64: 59-62.
- Tropov NA, Barzakovsky VP, Lapin VV (1969) State diagrams of silicate systems: A handbook. 1: L Nauka, p. 822.
- Vunain E, Mishra SB, Mishra AK (2017) Sol-gel based nanoceramic materials: preparation, properties and applications. Chapter-1. Nanoceramics: Fundamentals and Advanced Perspectives Springer International Publishing AG, p. 297.
- Rodrıguez MA, Aguilar CL, Aghayan MA (2012) Solution combustion synthesis and sintering behavior of CaAl2O4. Ceramics International 38(1): 395-399.
- Khomidov FG, Kadyrova ZR, Usmanov KL, Niyazova SM, Sabirov BT (2021) Features of the synthesis of aluminum-magnesium spinel by the sol-gel method. Glass and Ceramics 6: 48-52.
- Kocjan A, Pouchly V, Shen Z (2015) Processing of zirconia nanoceramics from a coarse powder. J Eur Ceram Soc 35(4): 1285-1295.
- Choi SW, Hong SH (2010) Size and morphology control by planetary ball milling in CaAl2O4:Eu2+ phosphors prepared by pechini method and their luminescence properties. Mater Sci Eng 171(1-3): 69-72.
- Aitasalo T, Holsa J, Jungner H, Lastusaari M, Niittykoski J (2006) Thermoluminescence study of persistent luminescence materials: Eu2+- and R3+- doped calcium aluminates, CaAl2O4: Eu2+, R3+. J Phys Chem 110(10): 4589-4598.
- Zhao C, Chen D (2007) Synthesis of CaAl2O4:Eu, Nd long persistent phosphor by combustion processes and its optical properties. Mater Lett 61: 3673-3675.
- Park K, Woo JP, Abdul Hakeem D (2016) Effect of alkaline metal ions on the photoluminescence properties of Eu3+-doped Ca3Al2O6 Journal of Rare Earths 34(12): 1193-1198.
© 2022 Kadyrova ZR. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






