- Submissions

Full Text
Aspects in Mining & Mineral Science
Hydrogen Fuel from Water Splitting – Green Renewable Energy
Nazeer A, Ahmad F and Ahmad S*
508, BWT, Eros Garden, Charmwood Village, Faridabad, Haryana, India
*Corresponding author: Ahmad S, 508, BWT, Eros Garden, Charmwood Village, Faridabad, Haryana, India
Submission: August 01, 2022;Published: August 16, 2022

ISSN 2578-0255Volume9 Issue4
Opinion
The current method of producing hydrogen is via Steam-Methane Reforming (SMR) in which hydrogen is produced by reacting steam with methane from natural gas under high pressures and temperatures. Despite the majority of the technologies of the hydrogen production through processes involving carbon emission, the alternative methods of hydrogen production are now catching up in the race of development. The methods to produce hydrogen through water electrolysis are becoming more desirable and cost-effective. For these technologies to become cost effective, various associated barriers must be surmounted to enhance their adaptability. These barriers generally come from regulatory, technical, and economical considerations [1]. Growth and market penetration of water electrolysis technologies that use renewable resources to produce hydrogen, attempts were made to analyse the total impact and published in the ‘Guidehouse Insights Report’. The areas identified include Alkaline Electrolysers (AELs), Proton Exchange Membrane (PEM) electrolysers, Solid Oxide Electrolysis Cells (SOECs), and Anion Exchange Membrane (AEM) electrolysers. This report presented a forecast capacity and revenue growth for these electrolysers globally [1]. Basics Economically viable commercial production of high purity H2 at affordable cost is currently being targeted to take care of the clean and green renewable source of energy in comparison to the other resources already in use. There are several methods as mentioned above already to produce hydrogen but the simplest one is through electrolysis in which the hydrogen and oxygen gases are produced as expressed in simple equation given below.
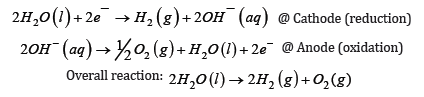
The major problem lies in optimising the catalysts used at the electrode and the way redox reactions are accomplished at minimum overpotential.
A simple process with huge impact!
According to a recent market research projection, growth of ~8,000% is expected in producing 100GW capacity of hydrogen electrolysers annually by 2031. The CAG (Compound Annual Growth) 62.6% is expected to lead the way in Europe as highlighted in the Guidehouse Insights report [2]. The annual manufacturing capacity will grow from about 1.3GW at the end of this year to 104.6GW by 2031- an increase of almost 8,000%, with a Compound Annual Growth Rate (CAGR) of 62.6%, the US-based company stated in its study, Market Data: Electrolysers. While the 104.6GW figure is much higher than the 47GW of electrolysers expected to be manufactured annually (by 2030) by US, it is still well below the 180GW foreseen to be in use by the end of the decade under the International Energy Agency’s (IEA) Announced Pledges Scenario and even further behind the 850GW to meet the net-zero emissions by 2050 as recommended by IEA. Around 14 GW-scale hydrogen generating electrolysers have already been announced to date - adding up to 26GW - by the manufacturers namely: Thyssenkrupp (5GW, Germany), ITM Power (5GW, UK), Plug Power (1GW in US; 1GW in South Korea; 2GW in Australia), John Cockerill (1GW in France; 2GW in India, in conjunction with Greenko), Siemens Energy (1GW in Germany), Cummins (1GW in Spain, with Iberdrola; and 1GW in China, with Sinopec), Nel (2GW, Norway), Ohmium (India, 2GW), McPhy (France, 1GW) and Sunfire (Germany, 1GW). It is quite clear that the electrolyser manufacturing market will be led by Europe, followed by Asia Pacific, then North America, Latin America and the Middle East and Africa, as discussed in the report. This gives a fairly clear picture of the development expected to take place in the hydrogen generation sector [2]. Global market leader - John Cockerill (Belgium) has announced to build 2GW H2 electrolyser factory in India with local renewables energy developer in partnership with Greenko Group [3].
Electrolysis – self-ionization process across a nanogap
The phenomenon of pure water (electrolyte free) electrolysis has been explained using deep-sub-Debye-length nanogap conceived in an electrochemical cell. When the gap between cathode and anode is smaller than Debye-length (i.e., 1 micron in pure water, around 220nm in distilled water), the double layer regions formed at the two electrodes overlap producing uniformly high electric field in the gap region. This kind of high electric field particularly facilitates the ion transport inside the water resulting into the self-ionization of water to keep the whole reaction continuing. In this case, the two half-reactions are coupled together and limited by electron-transfer steps and electrolysis current saturates with further reducing the electrode distance [4].
Small scale electrolyser and commercial plants
The simplest form of Hofmann voltameter used for water electrolysis consists of three upright cylinders joined at the bottom. The inner cylinder is open at the top to allow the addition of water and the electrolyte. A platinum electrode is placed at the bottom of each of the two side cylinders, connected to the positive and negative terminals of a battery. While current runs through the Hofmann voltameter, gaseous oxygen forms at the anode and gaseous hydrogen at the cathode (negative). Each gas displaces water and collects at the top of the two outer tubes available for collection via a stopcock. The industrial electrolysers are in fact similar to Hofmann voltameters, with complex platinum plates or honeycombs as electrodes. Generally, the only time hydrogen is intentionally produced from electrolysis is for the specific point of use application. The vast majority of hydrogen produced from hydrocarbons contains traces of carbon monoxide among other impurities. The carbon monoxide is detrimental to various systems called fuel cells. In high-pressure electrolysis the output is compressed hydrogen at 12-20MPa (1740-2900psi). By pressurising the hydrogen in the electrolyser, the need for an external hydrogen compressor is avoided; the average energy consumption for internal compression is around 3% [5].
Some critical component - PEM
A Proton-Exchange Membrane (PEM) is a semipermeable membrane meant for conducting protons and acting as an electronic insulator and reactant barrier (e.g., to oxygen and hydrogen gas). PEMs are either pure polymer or composite membranes with other materials embedded in a polymer matrix. Commercially available PEM materials is the fluoropolymer Nafion from DuPont. There are many other structural motifs used to make ionomers for PEMs. Many use polyaromatic polymers, while others use partially fluorinated polymers. PEMs are characterized by proton conductivity, methanol permeability, and thermal stability. PEM fuel cells use a thin plastic film, which is permeable to protons when it is saturated with water, but it does not conduct electrons [6-9]. Alternate technology suggested was to be using Supercritical Water Electrolysis (SWE) wherein water in a supercritical state needs less electrical energy to split the water bonds of hydrogen and oxygen, therefore enhancing the efficiency and reducing the costs. The increased temperature (>375 °C) reduces thermodynamic barriers and increases kinetics, improved ionic conductivity over liquid or gaseous water, reduces the ohmic losses. Benefits include improved electrical efficiency, >220 bar pressurised delivery of product gases, ability to operate at high current densities and low dependence on precious metals for catalysts. No commercial SWE equipment exists, however there are companies attempting to commercialise this version [10].
Numerous studies have been conducted recently to use non noble metals as catalyst in pure as well composite forms to reduce the cost of electrolysers. For improving specific surface areas of the catalysts nanostructured materials like NPs, nanorods, and nanosheets of metal, and metal-oxides have also been examined to improve the photocatalytic efficiencies for low-cost solutions. A variety of proposals have been worked out using DFT simulations, material syntheses and design of heterostructures using semiconducting nanosheets of different species to reduce the overpotentials. These categories of photocatalysts are expected to remove the application of external power source to initiate water electrolysis. Most of these recent investigations are striving hard to design doped, 2D-composites and heterostructures for reducing the overpotential as discussed by several research groups where both the components of cell assembly have been considered individually as well as simultaneously by invoking the principles of 2D- semiconductors offering tunability of location specific energy gaps to control the separations of the photogenerated electronhole pairs and their recombination for making them available at active sites on the surface to participate in redox reactions. With all these fundamental studies currently being conducted the commercial exploitation is still very far off. Still, the findings are worth considering as discussed in the present publications [11-14].
Ongoing commercial electrolyser development
High-temperature electrolysis (steam electrolysis) is currently being investigated for water electrolysis with a heat engine. High temperature electrolysis is better than room-temperature electrolysis because some of the energy is supplied as heat, which is cheaper than electricity, and because the electrolysis reaction is more efficient at higher temperatures. Water electrolysis known since long should be made available on the GW scale for meeting the energy demand within the energy transition towards renewable hydrogen. However, currently available electrolysers are still with low-automation processes resulting higher costs and low production capacity. With this background, a project named H2Giga was launched to advancing the industrialisation of the water electrolysis for the production of ‘green’ hydrogen by using renewable electricity sources. The main target is the industrial production of the electrolysers with partners involved in the manufacturing technologies, so that the entry of hydrogen market is accelerated. DECHEMA eV was involved in coordinating research and industry partners for implementing green hydrogen production, to leverage synergies to overcome the hurdles. The goal of H2Giga is to lay the foundation for competitive production of green hydrogen on a gigawatt scale, so that this energy carrier becomes available and affordable within the energy transition. European Institute for Energy Research (EIFER) as the project lead within the high temperature water electrolysis technology has been involved in the development of cell and stack modules for understanding of the degradation mechanism affecting the reliability of the system starting from April 2021 up to March 2025. The partners are from research and Industry with Kerafol GmbH, Karlsruhe Institute of Technology, DLR, Fraunhofer IKTS, Xenon GmbH, Kontron AS GmbH, ZBT, Fraunhofer IPA and others with a total fund of 1.5M€. The expected outcome is a knowledge advances in terms of degradation in the three technologies as well as the contribution to cell/stack performance and durability.
In spite of various barriers of progress as highlighted in brief and discussed in the ensuing publications in more detail the commercial production of high purity H2 for hundreds of GW capacity electrolysers are already declared to be introduced into the market. The introduction of nano photocatalysts will make a large difference once the right kind of composition, structural features and engineered material ban gaps will play significant role in meeting the ultimate goal of zero emission. Direct conversion of sea water into hydrogen fuel is worth pursuing in more detail due to its futuristic relevance [14,15].
References
- https://guidehouseinsights.com/reports/market-data-electrolyzers
- https://www.rechargenews.com/energy-transition/8-000-growth-more-than-100gw-of-hydrogen-electrolysers-to-be-produced-annually-by-2031/2-1-1201444
- https://www.rechargenews.com/energy-transition/global-market-leader-to-build-2gw-hydrogen-electrolyser-factory-in-india-with-local-renewables-developer/2-1-1200867
- Wang Y, Narayanan SR, Wu W (2017) Field-assisted splitting of pure water based on deep-sub-debye-length nanogap electrochemical cells. ACS Nano 11(8): 8421-8428.
- Ghosh PC, Emonts B, Janßen J, Mergel J, Stolten D (2003) Ten years of operational experience with a hydrogen-based renewable energy supply system. Solar Energy 75(6): 469-478.
- https://www.eifer.kit.edu/h2giganews/
- Alternative Electrochemical Systems for Ozonation of Water.
- Yang Z, Coutinho D, Feng F, Ferraris JP, Balkus KJ (2004) Novel inorganic/organic hybrid electrolyte membranes. Prepr Pap Am Chem Soc Div Fuel Chem 49(2): 599.
- New Proton Exchange Membrane Developed.
- Developing the world's first high pressure, ultra-efficient electrolyser.
- You B, Sun Y (2018) Innovative strategies for electrocatalytic water splitting. Accounts of Chemical Research 51(7): 1571-1580.
- Jiao Y, Zheng Y, Jaroniec M, Qiao SZ (2015) Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem Soc Rev 44(8): 2060-2086.
- Chen L, Shi J (2018) Chemical-assisted hydrogen electrocatalytic evolution reaction (CAHER). Journal of Materials Chemistry A 6(28): 13538-13548.
- Veroneau SS, Hartnett AC, Thorarinsdottir AE, Nocera DG (2022) Direct seawater splitting by forward osmosis coupled to water electrolysis. ACS Appl Energy Mater 5(2): 1403-1408.
- New 2D material could 'revolutionise hydrogen fuel from sunlight and water’.
© 2022 Ahmad S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






