- Submissions

Full Text
Aspects in Mining & Mineral Science
Preliminary Indications of Resistance to Hydrogen Embrittlement in Palladium-Copper Alloys of Copper Content 30–50 Weigh Percent
Andrew Craft*
Department of Chemistry, University of Hartford, West Hartford, USA
*Corresponding author: Andrew Craft, Department of Chemistry, University of Hartford, West Hartford, CT 06117, USA
Submission: April 13, 2022;Published: April 22, 2022

ISSN 2578-0255Volume9 Issue1
Abstract
Tensile strength and elongation characteristics have been determined for a series of palladium copper alloys that vary in copper content from 30 weight percent to 50 weight percent. A comparison of the elongation (ductility) properties of alloy specimens heat treated in vacuum to those exposed to hydrogen indicate that hydrogen exposure has little, if any, impact on the alloys. This finding provides preliminary evidence that the alloys have the ability to resist the damaging effects of hydrogen embrittlement.
Keywords: Palladium; Copper alloys; Metals; Hydrogen; Fabrication
Introduction
Hydrogen embrittlement is the bane of many technologically relevant processes that involve hydrogen. Many metallic materials are, to some degree, susceptible to hydrogen embrittlement. High-strength steels are prone to hydrogen embrittlement, as are zirconium alloys that are used in nuclear reactors. The high strength-to-density characteristics of titanium and its alloys can be negatively impacted by hydrogen embrittlement. The use of transition metals and alloys as hydrogen storage media or as hydrogen purification materials can be significantly compromised by the occurrence of hydrogen embrittlement. Palladium possesses many of the desirable properties to function as an effective hydrogen purification material – it has a high storage capacity for hydrogen; it has fast uptake and desorption of hydrogen; it absorbs and desorbs hydrogen under non-extreme conditions of temperature and hydrogen pressure; it is highly selective to hydrogen gas during permeation by gas mixtures. Unfortunately, all these desirable properties are offset by the severe hydrogen embrittlement suffered by palladium. Rebeiz and Craft have thoroughly characterized the hydrogen embrittlement in palladium [1]. Palladium-silver alloys have been found to be less susceptible to hydrogen embrittlement than pure palladium and have become one of the primary materials used in the fabrication of hydrogen purification membranes. The superior mechanical properties of palladium-silver alloys compared to palladium have been reported by Jimenez et al. [2].
Evidence is mounting that palladium-copper alloys may provide superior performance than palladium-silver alloys as hydrogen purification membranes, though their susceptibility to hydrogen embrittlement has not been thoroughly characterized quantitatively. DiMauro et al. [3] have recently reported on hydrogen embrittlement in palladium copper alloys with copper contents up to 25 weight percent [3]. The most attractive palladium-copper alloy for hydrogen purification applications contains ~40 weight% copper. Thus, the need exists to extend the characterization of hydrogen embrittlement in palladium-copper
alloys beyond the 25-weight percent copper maximum of the
DiMauro et al. [3] study. The present short communication reports
some preliminary results of a study on the mechanical properties
of palladium-copper alloys that compositionally vary from 30–50
weight percent copper.
Materials and Methods
Palladium-copper (99.9% pure basis metal) foils (ACI Alloys, San Jose, CA, USA) of 0.25mm thickness were used in this study. Details on the cleaning, heat treatment, and hydrogen exposure treatments of the palladium-copper specimens can be found in the study by DiMauro et al. [3]. Tensile tests were carried out, on both vacuum-annealed and hydrogen-cycled specimens, using an Instron Series IX Automated Materials Testing System (Instron Corporation, Norwood, MA, USA). Tensile stress–strain tests were performed at a constant elongation rate of 1.27mm/min. Yield strength and elongation at failure were determined by computer analysis of the stress-strain curves.
Results and Discussion
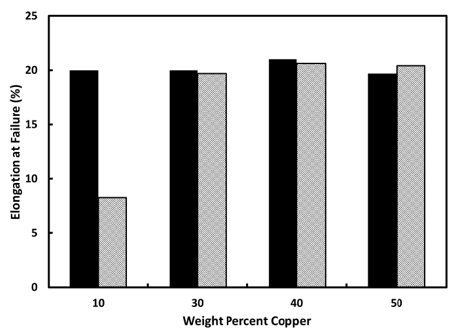
Tensile stress-strain tests were conducted on palladium-copper specimens that had been annealed in vacuum under conditions that allow recovery to a nearly defect-free state. The strength and elongation properties of these specimens establish the baseline values against which the effects of hydrogen exposure can be characterized. Identical stress-strain tests were conducted on vacuum-annealed specimens that had been exposed at 323K to hydrogen gas at 1atm (1.01x105Pa) pressure, followed by complete desorption of all the hydrogen absorbed during hydrogen exposure. The yield and ultimate strength values for both vacuum-annealed and hydrogen-cycled alloys show the expected increases associated with solid solution strengthening as the copper content increases. When strength values for a given alloy are compared between the vacuum-annealed and hydrogen-cycled specimens, virtually no difference is found. This indicates that hydrogen embrittlement has not manifest in the alloy. A more telling indicator of hydrogen embrittlement will be a loss of elongation (ductility) as a result of hydrogen exposure. Figure 1 shows the elongation at failure, for both vacuum-annealed and hydrogen-exposed specimens as a function of copper weight percent. For comparison, the values for palladium-copper (10 wt. % Cu) are shown in the figure [3]. The palladium-copper (10wt. % Cu) alloy illustrates the tell-tale sign of hydrogen embrittlement – a significant loss of ductility of the hydrogen-cycled alloy (grey bar in Figure 1) compared to the vacuum-annealed alloy (black bar in Figure 1). As can be seen in the figure, the alloys with copper weight percent between 30 and 50 do not show the dramatic loss of ductility that is emblematic of hydrogen embrittlement (as seen in the palladium-copper (10wt. %) alloy. These preliminary results bode well for the use of the palladium-copper (40wt.%) alloy as a hydrogen purification membrane. In addition to the attractive hydrogen diffusion and transport properties of this alloy, the preliminary results indicate that the alloy will not degrade due to hydrogen embrittlement. Obviously much more work is required to fully explore the resistance to hydrogen embrittlement by the palladium-copper alloys that are the basis of this brief report. But the preliminary results look promising.
Figure 1: Elongation at failure of vacuum-annealed (black bar) and hydrogen-cycled (gray bar) alloys as a function of weight percent copper.
References
- Rebeiz K, Craft A (2000) Tensile characteristics of palladium exposed to hydrogen (deuterium). ASCE J Energy Eng 126(3): 95-106.
- Jimenez G, Dillon E, Dahlmeyer J, Garrison Tr, Garrison Ty, et al. (2016) A comparative assessment of hydrogen embrittlement: Palladium and palladium-silver (25 weight% silver) subjected to hydrogen absorption/desorption cycling. Adv Chem Eng Sci 6: 246-261.
- DiMauro S, Legal G, Lubinsky C, Nadeau M, Tate R, et al. (2021) Strength, hardness, and ductility evidence of solid solution strengthening and limited hydrogen embrittlement in the alloy system palladium-copper (Cu wt.% 5–25). Hydrogen 2: 262-272.
© 2022 Andrew Craft. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






