- Submissions

Full Text
Aspects in Mining & Mineral Science
Liquid-Liquid Transitions due to Melting Temperatures of Residual Glassy Phases Expected in Pt57Cu23P20
Robert F Tournier*
National Institute of Applied Sciences of Toulouse, University of Grenoble Alpes, France
*Corresponding author: Robert F Tournier, UPR 3228 National Center for Scientific Research, National Laboratory of Intense Magnetic Fields, European Magnetic Field Laboratory, National Institute of Applied Sciences of Toulouse, University of Grenoble Alpes, F-31400 Toulouse, France
Submission: March 07, 2022;Published: March 15, 2022

ISSN 2578-0255Volume8 Issue5
Abstract
Bulk glasses are obtained by quenching melts of various compositions. The initial temperature is chosen above the liquidus temperature (T1) of materials assuming that the melt is homogenous. Glassy items are formatted above the glass transition temperature Tg below a crystallization temperature called Tx. First-order transitions at Tx transform melts into composites containing crystals and new glassy phases, melting at temperatures (Tn+) much higher than (T1). These first-order transitions were studied at Caltech Pasadena by cooling and heating a melt of Pt57Cu23P20. A recent model predicting the formation enthalpies of these new composites at Tx and the melting temperatures (Tn+) of glassy fractions is applied to this alloy.
Keywords: Crystallization; Liquid; Temperature; Transitions
Introduction
A detailed study of first-order transitions, occurring in the melt Pt57Cu23P20 during cooling and heating below its liquidus temperature, was recently published by J H Na et al. [1]. A theoretical description was proposed, considering that all these transitions lead to a full crystallization even far below the melting temperature. Another interpretation of these results is proposed in this paper in the light of a recent discovery of complementary melting temperatures of multiple glass phases surviving in glass-forming melts above the melting temperature [2,3].
The glass transition in Pt57Cu23P20 melt occurred at Tg=505K, the solidus melting temperature at Ts=Tm=823.8K and the liquidus temperature at T1=827.9K. In spite of an overheating temperature higher than 1173K, Fiftheen thermal cycles were necessary to attain the highest undercooling rate ()@286TKΔ. First-order transitions were observed near Tg, after annealing the glass during 15 hours at 503K. The melting heat was 68.5J/g [1].
The first objective, in this paper, is to attribute the first-order transitions near Tg to the formation of glacial phases as already observed in triphenyl phosphite, d-mannitol, n-butanol and water [4-11]. A non-classical model of homogeneous nucleation, built from the knowledge of Tg and Tm, had explained the glacial phase formation by considering that the first-order transition was driven by the entropy of a new phase called Phase 3 [12,13]. The existence of entropically-driven transitions in Al2O3-Y2O3 system was recognized, for the first time, by Assland & McMillan [14] while the formation of Phase 3 was proposed by heating supercooled water [15,16].
Phase 3 obtained by heating the melt from the glassy state, has been attributed to the presence, above Tg, of residual bonds in all glasses, melted at temperatures Tn+ higher than Tm [17]. The non-classical model of homogeneous nucleation was recently completed, leading to the prediction of Phase 3 transformations in glassy phases at new temperatures Tx which are driven by enthalpy [3]. The melt above Tg is viewed as containing residual bonds (after configurons formation [18-21]) at a critical threshold up to a first-order transition at T=Tx [17]. Above Tx, new glassy Phases 3 of lower enthalpy, could be formed with a glass transition temperature Tg’ equal to (2Tm-Tg) = 1142.6K which remains virtual because (Tg’) is higher than their melting temperatures (Tn+) [3].
First-order transitions occurring near Tg=505K in Pt57Cu23P20 were varying between Tx=535.6K and 548.8K during heating. These transition temperatures slightly increased because the liquid enthalpy, relaxed at low heating rates, induced entropy and enthalpy variations due to the long annealing (15 hours) initially applied at Tg and to very low heating rates (0.2-10K/mn) up to Tm [1] Figure 1. All latent heats of transitions at Tx, including those observed by under cooling, were attributed to the melt crystallization which increased from 20J/g near Tg to 68.5J/g at Tm [1] in contradiction with our description of a new phase formation which is entropically driven.
Figure 1: First-order transition at Tx=548.8K during heating. Observed in [1] Figure 1E. T0m=389.9K. Increase of the enthalpy after 15 hours of annealing at Tg=505K. Melting temperature Ts=Tm=823.8K. Enthalpy coefficient (-0.34418) resulting from the transition driven by the entropy at Tx. Transformation of Phase 3 in a new glass at Tx=548.8K with an enthalpy coefficient during the first heating and melting at Tm=823.8K.
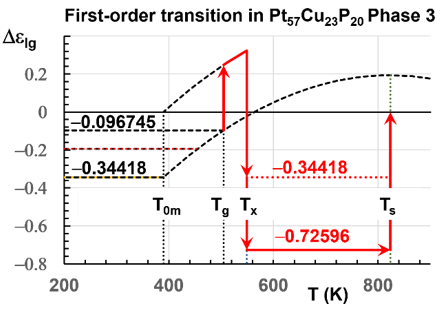
The second objective is to determine when these findings are compatible with a glass formation, following an increase of residual bond fraction at the transition at Tx, driven by the configuron entropy toward a new Phase 3 of lower enthalpy. These bonds may disappear at temperatures Tn+ higher than Tm as already observed in many glasses and predicted by non-classical nucleation equation [17,22,23]. They give rise to a glass phase fraction in the melt above Tx. The maximum fraction of atoms involved into the glass phase is defined by the latent heat inducing transition at Tx.
The third objective is to predict the multiple melting temperatures (Tn+), higher than Tm, of these new glassy fractions and the total melting heat of Pt57Cu23P20, including the additional latent heat recovered at Tn+.
2-Practical Equations Applied to Liquid Pt57Cu23P20 Enthalpies
There are 3 fragile liquid states having enthalpies equal to ε lsHm , ε gsHm and Δε l gHm where Hm is the melting enthalpy [24]:
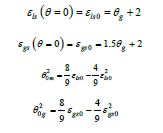
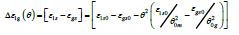
where ( ) 0m θ and ( ) 0 g θ are the reduced and calculated Vogel- Fulcher-Tammann (VFT) temperatures higher than (-2/3) for fragile liquids. The enthalpy coefficients devoted to Pt57Cu23P20 are:
Liquid 1
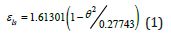
Liquid 2
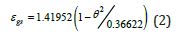
Phase 3
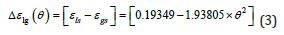
3-First-Order Transitions Driven by the Entropy of Phase 3 during the First Heating
The singular enthalpy coefficients 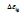 The singular enthalpy coefficients
The singular enthalpy coefficients 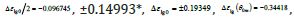 and (-0.33342=-
0.14933-0.19349) and expected to correspond to various
percolation thresholds [3,17,25]. The entropy variation of Phase 3
from the solidus melting temperature (Ts=Tm=823.8K) down to the
Kauzmann temperature TK=364.43K is equal to (-1/Tm) assuming
that Hm=1J/g. The entropy variation of Phase 3 in Eq. (4), up to a
temperature T, starts from the temperature TK:
and (-0.33342=-
0.14933-0.19349) and expected to correspond to various
percolation thresholds [3,17,25]. The entropy variation of Phase 3
from the solidus melting temperature (Ts=Tm=823.8K) down to the
Kauzmann temperature TK=364.43K is equal to (-1/Tm) assuming
that Hm=1J/g. The entropy variation of Phase 3 in Eq. (4), up to a
temperature T, starts from the temperature TK:
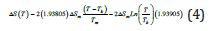
For T=Tx, ΔS (TX) is equal to the entropy change produced by the
first-order transition which is equal to 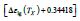 /Tx=0.47928/
Tx=0.0008719J/K/g at Tx=548.8K with Hm=1J/g, Δε lg ( TX )= 0.13342
and TK=364.63K. The enthalpy coefficient inducing the first-order
transition is equal to (-0.34418) deduced from
/Tx=0.47928/
Tx=0.0008719J/K/g at Tx=548.8K with Hm=1J/g, Δε lg ( TX )= 0.13342
and TK=364.63K. The enthalpy coefficient inducing the first-order
transition is equal to (-0.34418) deduced from 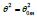 in Eq. (1)
and
in Eq. (1)
and 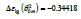 in Eq. (3). The measured latent heat at Tx is
(49J/g=0.71533×Hm=0.71533×68.5J/g) [1] and the latent heat
coefficient, being higher than (0.47928), is enhanced by adding
the singular enthalpy coefficients (0.096745+0.14993), leading
to the theoretical value (0.72596×68.5=49.7J/g). This glassy
phase, having a very low enthalpy, cannot exist above Tm with a
melting temperature Tn+=1.72596Tm, which would be higher than
Tg’=1.38699Tm=1142.3K. This glassy phase is melted at Tm=823.8K.
in Eq. (3). The measured latent heat at Tx is
(49J/g=0.71533×Hm=0.71533×68.5J/g) [1] and the latent heat
coefficient, being higher than (0.47928), is enhanced by adding
the singular enthalpy coefficients (0.096745+0.14993), leading
to the theoretical value (0.72596×68.5=49.7J/g). This glassy
phase, having a very low enthalpy, cannot exist above Tm with a
melting temperature Tn+=1.72596Tm, which would be higher than
Tg’=1.38699Tm=1142.3K. This glassy phase is melted at Tm=823.8K.
First-order transitions temperatures lower than Tx=548.8K
are calculated with Eq. (4) and temperature (TK) equal to 364.4K
in agreement with experimental latent heats. For Tx=535.6K,
the measured latent heat (38.7J/g) corresponds to a fraction
(38.7/68.5=0.56496) of Hm and to an entropy of transition
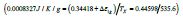 TK=364.63K and Δε lg = 0.1018 . The
singular latent heat coefficient (0.096745) is added to (0.44598)
to attain the theoretical value (0.54273) during the transition
and a latent heat of 37.2J/g. For Tx=Tg=505K, the highest relaxed
entropy after isothermal annealing could be
TK=364.63K and Δε lg = 0.1018 . The
singular latent heat coefficient (0.096745) is added to (0.44598)
to attain the theoretical value (0.54273) during the transition
and a latent heat of 37.2J/g. For Tx=Tg=505K, the highest relaxed
entropy after isothermal annealing could be 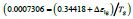 for Δε lg = 0.02482 and TK=364.63K and the relaxed enthalpy
(0.369×68.5=25.3J/g) [1]. The various values of Δε lg depend on the
enthalpy which is relaxed during annealing at Tg and slow heating
up to Tx. All these enthalpy changes are driven by the entropy of
Phase 3 without any crystallization.
for Δε lg = 0.02482 and TK=364.63K and the relaxed enthalpy
(0.369×68.5=25.3J/g) [1]. The various values of Δε lg depend on the
enthalpy which is relaxed during annealing at Tg and slow heating
up to Tx. All these enthalpy changes are driven by the entropy of
Phase 3 without any crystallization.
4-First-Order Transitions Driven by the Enthalpy of Phase 3 during Supercooling
Temperatures of undercooling are predicted using the nonclassical nucleation model leading to nucleation temperatures ( ) θ n− in Liquid 2 defined in Eq. (5) [26]:
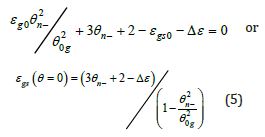
where Δε is equal to singular values of the enthalpy coefficient
of Phase 3. The initial enthalpy coefficient Δε lg at the supercooling
temperature would be much higher than (-0.34418) after 15
Differential Scanning Calorimetry (DSC) cycles at 0.17K/s from
1173K to 373K [1] and equal to the enthalpy coefficient ( Δε lg=0 ) of
homogeneous liquid. The transitions during undercooling occur
between 635K and 689K starting from the cooling enthalpy ( Δε lg=0 ) as
shown in Figure 2, using Eq. (5), Δε = 0.096745 , 0.19349, 0.246675,
and 0.34418 respectively and  and
and 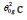 given by Eq. (2). These new transitions lead to new glassy Phases 3 which are accompanied
by crystallization of the other liquid fraction.
given by Eq. (2). These new transitions lead to new glassy Phases 3 which are accompanied
by crystallization of the other liquid fraction.
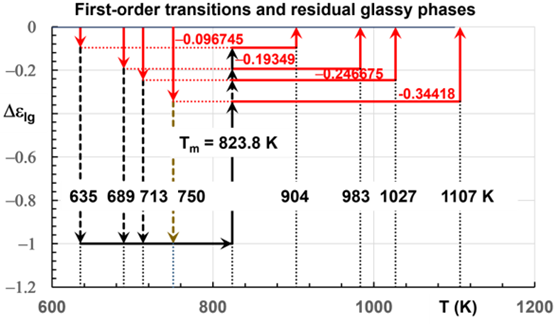
Figure 2: First-order transitions starting from the homogeneous liquid state ( ) Δε lg = 0 . Nucleation of Phase 3 leading to crystallization for various undercooling rates (from 74 to 189K). The enthalpy change starts from 0 toward four singular values of Δε lg in Eq. (5) equal to -0.096745, -0.19349, -0.246675 at 635, 689K, 713K and 750K leading to the formation of glassy phases 3 and to spontaneous crystallization. Melting temperatures Tn+=903.5K, 983.2K, 1027K and 1107.3K. Transitions at Tx described in [1] Figure S4c. The crystallization heat is equal to hcr for .
There is an experimental jump of undercooling rate between 689K and 713K corresponding to that of singular values from (-0.19349) to (-0.246675) in rough agreement with a group of 25 undercooling rates between 140 and 189K following 15 previous thermal cycles [1] Figure S4c. The residual glassy phases, in these conditions, are formed at temperatures Tx=635, 689, 713K and 750K through first-order transitions inducing crystallization and melted after reheating at Tn+=903.5K, 983.2K, 1027K and 1107K respectively. The lowest enthalpy of Phase 3 is found again for Δε = 0.34418 without being accompanied by supplementary enthalpy coefficients at Tx=750K leading to Tn+=1107K.
The nucleation of crystallization, at weak undercooling rate, starts in Figure 3 from Δε lg = −0.34418 instead of zero in Figure 2. A first-order transition is expected at Tn+=1107K in spite of an overheating at 1173K. Eq. (5) is applied for (ε gs0 = 1.41952 − 0.34418 = 1.07534) , (θo2g = 0.36622) as in Eq. (2), (Δε = 1− 0.34418 = 0.65582) and leads to a nucleation temperature (742.1K) near the experimental value (737.4K). All measured latent heats [1] were viewed as “crystallization” enthalpies including those of glassy fractions from Tg to Tm and were represented by Eq. (6) including the latent heats of Phases 3 at Tx<548.8K:
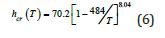
The “crystallization” heat hcr at Tm=823.8K was 69.2J/g, applying Eq. (6). The highest crystallization temperature occurred at 737.4K instead of 742.1K with a “crystallization” heat (hcr=67.82J/g). This homogeneous nucleation temperature is obtained with Eq. (5) for ε gs0 = 1.06795 instead of 1.07534, and Δε = 0.64883 . The initial enthalpy (Δε lg) before supercooling is (1.06795-1.41952=-0.35157) instead of (-0.34418) corresponding to a “crystallization” heat (67.82J/g) deduced from Eq. (6). The crystallization at 737.4K starts from an undercooling rate (86K) during the first thermal cycle and at 720.9K with an undercooling rate (103K) for the 15th thermal cycle and a “crystallization” heat (67.35J/g), weakly reduced (67.82−67.35=0.47J/g) and associated with a weak increase of Δε up to 0.3585. This result confirms the existence of a second group of undercooling rates between 86 and 103K inducing crystallization during the first DSC cycles [1]. The glassy Phase 3 would be formed and melted through a first-order transition at 1107.3K during the first thermal cycles as shown in Figure 3 and as already observed in Cu46Zr46Al8 [3,27].
Figure 3: Nucleation of crystallization at weak undercooling rate (82K) from Δε lg = −0.34418 . The enthalpy coefficient change starting from -0.34418 toward -1, the crystal enthalpy coefficient with in Eq. (5). Transitions at Tx described in [1] Figure S4c. The crystallization heat is equal to hcr for Δε lg = −1 .
The “crystallization” heat hcr during supercooling, including the latent heat of glassy Phase 3, depends on singular value (Δε ) . For Δε = 0.096745 and Tx=635K, the latent heat hcr is equal to −62.9J/mole in Eq. (6). For Δε = 0.19349 , Tx=689K, hcr=−66.1J/mole in Eq. (6). For Δε = 0.246675 , Tx=713K, hcr=−67.1J/mole as shown in Figure 2. The missing melting heats (0.096745Hm), (0.19349Hm), (0.246675Hm) and (0.34418Hm) of Phases 3 are recovered at Tn+=1.096745Tm, 1.19349Tm, 1.246675Tm and 1.34418Tm respectively. The enthalpy of the crystallized fraction included in the crystallization heat hcr is weakly reduced from Hm=68.5 to 62.9J/g when the elapsed time in the undercooling rate ΔT increases. This phenomenon was observed after annealing a glass-forming melt at temperatures lower than Tx [3,28].
A measurement of melting heat Hm must eliminate the glassy fraction of configurons. The melt must be overheated above Tg’ if possible, annealed above Tm to induce new growth nuclei able to crystallize the melt at Tm without undercooling. Eq. (5) predicts here a nucleation temperature of complete crystallization at 844K with Δε = 1. The glassy fractions have been melted in Pt57Cu23P20 leading to Hm=68.5J/g [1].
Conclusion
First-order transitions observed near Tg=505K in Pt57Cu23P20 at various temperatures (Tx), varying from 535.6 to 548.8K, are entropically driven during heating and lead to the formation of glassy Phases 3 with a lower enthalpy at Tx without crystallization at these temperatures. Those observed by supercooling at homogeneous nucleation temperatures Tn-=Tx between 635 and 750K are driven by an increase of singular values of configuron enthalpies and lead to the formation of various Phases 3, being glassy from Tx to their melting temperature (Tn+). All these firstorder transitions are accompanied by crystallization.
The measured crystallization enthalpy hcr, equal to 68.5J/g, observed at the solidus temperature Ts, is complete. The total melting heat, including the latent heat expected at Tn+ above Tm, resulting from the melting of glassy Phase 3, which are formed at Tx by undercooling, is equal to hcr≤68.5J/g. The crystallization heat slightly decreases with the elapsed time in the undercooled state and the annealing time between Tg and Tx.
This work shows that glass-forming melts are composed of crystallized fractions or liquid fractions and glassy fractions, melting at temperatures Tn+ much higher than the crystal melting temperature.
The presence of glassy phases above the crystal melting temperature, after a first-order transition at Tx, obtained by undercooling below Tg and reheating, has for consequence that the crystallization heat must be measured by cooling the melt, if possible, from the second glass transition temperature (Tg’), and in all cases, by annealing it above Tm to grow new nuclei and crystallizing it without undercooling.
*A latent heat is expected at Tn+=1.14766×Tm in the absence of long annealing at Tg [17,23].
Acknowledgment
The author is grateful to M.I. Ojovan whose information’s about percolation of configurons helped to improve the paper.
References
- Na JH, Corona SL, Hoff A, Johnson WL (2020) Observation of an apparent first-order glass transition in ultrafragile Pt–Cu–P bulk metallic glasses. PNAS 117(6): 2779-2787.
- Tournier RF, Ojovan MO (2021) Prediction of second melting temperatures already observed in pure elements by molecular dynamics simulations. Materials 14(21): 6509.
- Tournier RF, Ojovan MI (2022) Multiple melting temperatures in glass-forming melts. Sustainability 14: 2351.
- Kivelson D, Kivelson SA, Zhao X, Nussinov Z, Tarjus G (1995) A thermodynamic theory of supercooled liquids. Physica A 219(1-2): 27-38.
- Ha A, Cohen I, Zhao X, Lee M, Kivelson D (1995) Supercooled liquids and polyamorphism. J Phys Chem Lett 100(1): 1-4.
- Miltenburg KV, Blok K (1996) Calorimetric investigation of a new solid phase in Triphenylphosphite. J Phys Chem 100(41): 16457-16459.
- Tanaka H, Kurita R, Mataki H (2004) Liquid-liquid transition in the molecular liquid triphenyl phosphite. Phys Rev Lett 92(2): 025701.
- Kobayashi K, Tanaka H (2016) The reversibility and first-order nature of liquid-liquid transition in a molecular liquid. Nature Comm 7: 13438.
- Zhu M, Wang JQ, Perepezko JH, Yu L (2015) Possible existence of two amorphous phases of D-Mannitol related by a first-order transition. J Chem Phys 142(24): 44504.
- Kurita R, Tanaka H (2005) On the abundance and general nature of the liquid-liquid phase transition in molecular systems. J Phys: Condens Matter 17: L293-L302.
- Bolshakov BV, Dzhonson AG (2005) On the number of amorphous phases in n-butanol kinetics of free radicals oxidation by oxygen in frozen n-butanol. J Non-Cryst Sol 351(5): 444-454.
- Tournier RF (2019) First-order transitions in glasses and melts induced by solid superclusters nucleated by homogeneous nucleation instead of surface melting. Chem Phys 524: 40-54.
- Tournier RF (2020) Homogeneous nucleation of phase transformations in supercooled water. Physica B: Con Mat 579: 411895.
- Assland S, McMillan PF (1994) Density-driven liquid-liquid phase separation with system Al2O3-Y2O3. Nature 369: 633-636.
- Tournier RF (2018) Predicting glass-to-glass and liquid-to-liquid phase transitions in supercooled water using completed classical nucleation theory. Chem Phys 500: 45-53.
- Tournier RF (2021) Encyclopedia of Glass Science, Technology, History, and Culture. In: Richet P (Ed.), Amorphous ices. Hoboken: Wiley & Sons, US, 1: 14.
- Tournier RF, Ojovan MI (2021) Undercooled phase behind the glass phase with superheated medium-range order above glass transition temperature. Physica B 602: 412542.
- Ojovan MI, Travis KP, Hand RJ (2007) Thermodynamic parameters of bonds in in glassy materials from viscosity temperature relationships. J Phys: Cond Matter 19: 415107.
- Ojovan MI, Lee WE (2010) Connectivity and glass transition in disordered oxide systems. J Non-Cryst Sol 356: 2534-2540.
- Ojovan MI (2013) Ordering and structural changes at the glass-liquid transition. J Non-Cryst Sol 382: 79-86.
- Ojovan MI, Louzguine Luzgin DV (2020) Revealing structural changes at glass transition via radial distribution functions. J Phys Chem 124(15): 3186-3194.
- Tournier RF, Ojovan MI (2021) Dewetting temperatures of prefrozen and grafted layers in ultrathin films viewed as melt-memory effects. Physica B 611: 412796.
- Tournier RF, Ojovan MI (2021) Building and breaking bonds by homogenous nucleation in glass-forming melts leading to three liquid states. Materials 14(9): 2287.
- Tournier RF (2014) Fragile-to-fragile liquid transition at Tg and stable-glass phase nucleation rate maximum at the Kauzmann temperature TK. Physica B 454: 253-271.
- Hasmy A, Ispas S, Hehlen B (2021) Percolation transitions in compressed SiO2 Nature 599: 62-66.
- Tournier RF (2016) Glass phase and other multiple liquid-to-liquid transitions resulting from two-liquid competition. Chem Phys Lett 665: 64-70.
- Zhou C, Hu L, Sun Q, Qin J, Brian X, et al. (2013) Indication of liquid-liquid phase transition in CuZr-based melts. Appl Phys Lett 103(17): 171904.
- Jiang J, Saksl K, Nishiyama N, Inoue A (2002) Crystallization in Pd40Ni40P20 glass. J Appl Phys 92: 3651-3656.
© 2022 Robert F Tournier. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






