- Submissions

Full Text
Aspects in Mining & Mineral Science
Ash from the Thermal Power Plant as a Raw Material for Ferrosilicon Production
Bagliuk GA*, Dubok VA, Kurochkin VD and Kurovskyi VY
Institute for Problems of Materials Science (IPMS) of the National Academy of Sciences of Ukraine, Ukraine
*Corresponding author: Bagliuk GA, Institute for Problems of Materials Science (IPMS) of the National Academy of Sciences of Ukraine, Ukraine
Submission: January 10, 2022;Published: February 16, 2022

ISSN 2578-0255Volume8 Issue4
Abstract
A qualitative and quantitative chemical analysis of samples of sludge from a thermal power plant obtained after magnetic separation has been carried out. It is shown that the main components of the material are iron and silicon (partially in the form of oxides). A technological scheme for the utilization of sludge is proposed, including the magnetic separation of slags and their subsequent processing by melting their mixture with sodium tetraborate. As a result of the study of the chemical and phase composition of the resulting alloy, it was found that it contains about 86.4% Fe, 10.6% Si and 0.9% C, which leads to the formation of the predominant phases Fe3Si and Fe3C in the alloy composition close to the composition of ferrosilicon - ferroalloy, which is widely used in the production of cast irons and high-quality silicon steels.
Keywords: Ash; Slag; Utilization; Ferroalloy; Iron; Silicon; Oxide; Ingot
Introduction
When coal is fired at thermal power plants, depending on the quality of coal and the method of combustion, mineral components are converted into ashes and slag, which are stored as waste products of energy production in ash dumps. At combustion temperatures ranging from 1500 to 1800 °C the coal components are disintegrating or melting. The composition of ash and slag consists of quartz grains and clay minerals, particles of vitreous material similar to volcanic glass, particles of newly formed minerals - mullite, magnetite, ferrosilicon and others. The characteristics of ash are different at different thermal power plants, because they depend on the composition of the mineral components of coal, the method of preparing fuel for combustion, the combustion technology, the flue gas cleaning system from ash, and the method of transporting ash. The ash dumping places of coal thermal power plants in the country stored tens of millions of tons of ash and slag, and the total area of land occupied by them amounts to thousands of hectares. These slag dumps of thermal power plants continue to increase annually by thousands of tons, and they generate a significant environmental threat to the surrounding regions [1,2]. The ashes, while accumulating in the dumps in significant volumes, may be of industrial interest as raw materials [2-6]. The most effective technology for utilization of slag and ash from thermal power plants is its use in the construction industry in the production of concrete as an inexpensive substitute for natural and man-made light aggregates [2,3,6]. There are also known examples of their use as fillers for asphalt and other road carpets.
Construction materials are the most obvious, but far from the only direction in the utilization of ash dumps. Ash and slag can be a valuable source of metals. Metals are found in coal as part of various minerals and organometallic compounds. When coal is burned, a significant part of it turns into ash. Technologies have been developed for extracting aluminum oxides from ash and slag materials as a raw material for the subsequent production of metallic aluminum [7,8]. In a much smaller volume, ash and slag wastes are used to extract iron-containing components. In this regard, the purpose of this work was to study the possibility of obtaining an industrially competitive technology of an iron-containing metallurgical product from ash and slag waste from thermal power plants.
Materials and Experimental Details
Samples from the Tripylska (Ukraine) thermal power plants ashes were taken as the base material. For the experiment, the ash component obtained after the magnetic separation of the slag was used. The powder mixture obtained after magnetic separation was mixed with sodium tetraborate (as reducing agent) and was melted in a refractory crucible of aluminum oxide in a protective argon atmosphere. The chemical composition of the ash and the alloy obtained from it was studied by emission spectral analysis (for the initial semi-quantitative determination of the composition of the main elements and impurities), chemical solution analysis and electron probe analysis. Their microstructure was studied using an XJL-17AT metallographic microscope and a JEOL Superprobe-733 scanning electron microscope. X-ray studies of the alloy were carried out in Co-Kα-radiation on a DRON-3 diffractometer. The experimental results were processed using Powdercell 2.4 program for the full-profile analysis of X-ray spectra. The Rockwell hardness of the obtained alloy was determined using a NOVOTEST TS-BRV hardness tester, and the microhardness of the constituent phases - using a PMT-3 microhardness tester.
The Results of the Experiments and their Discussion
Figure 1: Structure of the surface of the fraction of ball particles (a) and structure of iron (rust) scale fraction (b).
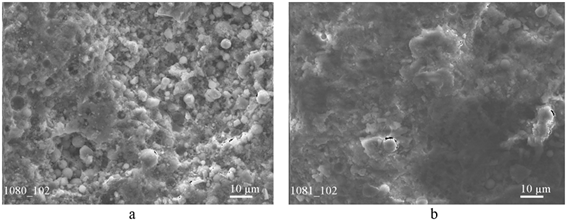
For investigation of the original ash, two components prevailing in the volume of waste were selected: fused metal inclusions in the form of balls with an average diameter of about 1.9mm and dispersed fire scale powder. The microstructures of the selected fractions are shown in Figure 1. As can be seen from (Figure 1a) on the surface of a ball with a diameter of 1.9mm there are both pores and small balls of different diameters - from 1 to 10μm. The separated scale fraction is distinguished by a significantly greater dispersion (particle size up to 1-2 microns) with individual spherical inclusions 2-5 microns in size. As it was shown by the results of a qualitative assessment of the chemical composition of the ash, carried out by the emission spectral analysis, the main components of the material are iron and silicon (partially in the form of oxides). In addition, small amounts of such elements as Al, Mg, K, Ca, Ti, Cu are found in the composition of the ash as reliably present. A characteristic feature of the isolated dispersed scale fraction is that it contains significantly more silicon, aluminum and calcium in comparison with the fraction of fused spherical particles (Figure 1b). The oxygen content in different fractions of the ash varies widely from 4.0 to 32.5%. The maximum amount of oxygen contains a very fine scale fraction. After averaging the fractions, the ash that underwent magnetic separation had the following chemical composition: Fe - 77±2%; Si - 10±3%; Al - 4±3%, K - 3±2%, Ti - 1.1±0, 5%, Ca, Mn, S - about 0.1% each, O2 - 5±3%.
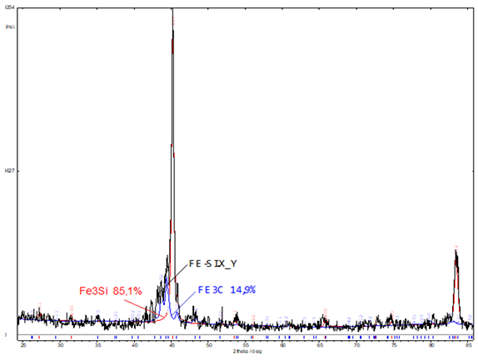
After heating the mixture of this powder with sodium tetraborate above the melting point of the mixture, a metal-like ingot and a layer of black slag were obtained. The resulting ingot has a fairly high hardness - 40 - 51 HRC. The microstructure of the ingot shown at Figure 2 consists of the dark dendrites phase and light phase - interdendritic liquation zones. The microhardness of dark dendrites of an ingot is – 4.33 - 4.67GPa, and interdendritic segregation zones (light) - 7.53 - 7.97GPa. There are some areas with microhardness of 11.45GPa too. Precision complex analysis of the chemical composition of the ingot metal gave the following results: iron - 86.4%, silicon - 10.6%, carbon - 0.93%, the other part of the ingot - 0.93%, oxygen - 0.16%, titanium - 0.17%. According to the spectral analysis, the ingot also contains Mg, Mn, Ni and V in an amount of about 0.1% each. The data of X-ray phase analysis of the ingot metal are shown in Figure 3. The phase composition, determined from these results: Fe3Si - 85,1% with lattice parameter a=0,5677±0,0005nm; Fe3C - 14,9 % (mass) with lattice parameters: a=0,5112nm; b=0,60879nm; c=0,5342nm.
Figure 2: Microstructure of metal ingot (etched): the dark phase - dendrites, light phase - interdendritic liquation zones.
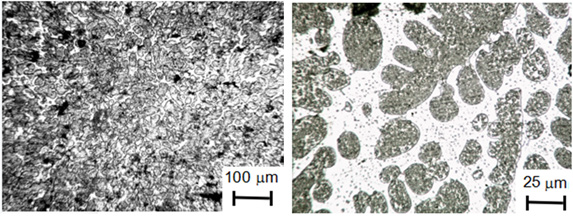
Figure 3: X-ray phase analysis of the ingot after melting.
Thus, the presented data show that the result of the application of the technological scheme, including the magnetic separation of slags from thermal power plants and their subsequent processing by melting their mixture with sodium tetraborate, is the production of a high-quality alloy close in composition to ferrosilicon - ferroalloy, which is widely used in the production of cast irons and high-quality silicon steels. In addition, given the high hardness of the resulting alloy, it is possible to independently use it as a wear-resistant material for the manufacture of parts operating under conditions of intense (including abrasive) friction and wear. Ferrosilicon also has a very high corrosion resistance in the environment of acids and alkalis; therefore, it is used in the manufacture of parts for equipment operating in corrosive environments. Taking into account that the essential part of the silicon impurities and the main part of the aluminum and calcium impurities passed (in the form of oxides) into the slag, its composition in this case is close to marl - a raw material widely used for producing of cement.
References
- Aleksandrova TN, Korchevenkov SA (2017) Ecological and technological aspects of ash and slag wastes utilization. Journal of Ecological Engineering 18(4): 15-24.
- Nisnevich M, Sirotin G, Eshel YT (2003) Environmental aspects of utilizing coal combustion by-products for production of lightweight concrete. The Twentieth Annual International Pittsburgh Coal Conference, Coal-Energy and the Environment, Pittsburgh, USA 1: 1-14.
- Blisset RS, Rowson NA (2012) A review of the multi-component utilization of coal fly ash. Fuel 97: 1-23.
- Menshov PV, Khlupin YV, Nalesnik OI, Makarovskikh AV (2014) Ash and slag waste as a secondary raw material. Procedia Chemistry 10: 184-191.
- Fomina EY, Artemova OS (2011) Investigation of the possibility of recycling ash and slag waste from thermal power plants by metallurgical methods. Mining Information and Analytical Bulletin 8: 273-277.
- Kosivtsova Y, Chalova K, Sulmana M, Yuri L, Tatiana N, et al. (2021) Use of ash and slag waste from thermal power plants as an active component of building materials. Chemical Engineering Transactions 88: 337-342.
- Sangita S, Nayak N, Panda CR (2017) Extraction of aluminium as aluminium sulphate from thermal power plant fly ashes. Transactions of Nonferrous Metals Society of China 27(9): 2082-2089.
- Gao Y, Liang K, Gou Y, Wei S, Shen W, et al. (2021) Aluminum extraction technologies from high aluminum fly ash. Reviews in Chemical Engineering 37(8): 885-906.
© 2022 Bagliuk GA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






