- Submissions

Full Text
Aspects in Mining & Mineral Science
Formation of Rhenium and Tantalum During Electrolysis of Distilled Water Using Tungsten Electrodes and the Expected Isotope Ratio
Kashchenko MP1,2, Pechorsky VI2, Kashchenko NM1*, Nikolaeva NV3 and Pushin VG1,3
1Ural Federal University named after the first President of Russia B N Yeltsin, Russia
2Ural State Forest Engineering University, Russia
3Mikheev Institute of Metal Physics, Ural Branch of RAS, Russia
*Corresponding author: Nadezhda M Kashchenko, Institute of Physics and Technology, Ural Federal University named after the first President of Russia B N Yeltsin, Yekaterinburg, Russia
Submission: December 23, 2021;Published: January 12, 2022

ISSN 2578-0255Volume8 Issue3
Abstract
The original installation for plasma electrolysis of water demonstrated, according to [1], an example of the implementation of low-energy nuclear reactions of the synthesis of chemical elements with low erosion of electrodes. That is, the initial material for the formation of chemical elements was water, and the product was a solid fraction in the form of a polymetallic powder.
Keywords: Copper; Chemical elements; Tungsten electrodes; Rhenium
Introduction
Copper was most often used as the electrode material. The synthesis of elements was associated with the emergence of a stable “plasmoid” between the electrodes spaced at a distance of (1-1.5) D, where D is the inner diameter of the hollow electrodes (D≤50mm). The flow-through version was used (water circulation). The emergence of a “plasmoid” was initiated by a triggering electric discharge in the transverse direction with respect to the flow of water. In [2], vertical tungsten rods were used as electrodes. The flow-through version was not used. The potential difference between the electrodes did not exceed 340V. Potash (K2CO3) was added to distilled water to increase conductivity. No significant erosion of the electrodes was observed, and the appearance of new elements was recorded on the surface of the electrodes. The list of publications confirming the formation of elements in reactions of low-temperature synthesis is currently quite extensive and is constantly being updated. The main problem is the interpretation of the results. A possible explanation of the synthesis mechanism requires an extension of traditional concepts. This expansion is possible [3,4] using the inferences of hadronic mechanics [5]. At the same time, for the further development of the theory and improvement of the technology of synthesis of elements, it is advisable to build up the experimental data base.
In this work, pulsed electric discharges were carried out in distilled water, and, in contrast to [1,6], not tubular, but rod-shaped tungsten electrodes with hemispherical tips were used. The starting potential difference between the electrodes was 1kV. The gap between the electrodes is ≈0.7mm. The electrodes in the working cell were placed horizontally. The excess pressure in the cell was released through the valve. The water level in the cell, as a rule, corresponded to the full coverage of the electrodes. The flow-through version (water circulation) was not used. After several electrical discharges and evaporation of the liquid, about a milligram of white powder was obtained, sufficient for microanalysis of the chemical
composition. In [7], the concept of a quasineutron is introduced as
a bound state of a proton and an electron that exists in a wide range
of spatial scales and corresponding energies. The publication [6]
emphasizes that the presence of quasineutrons should manifest
itself in the simplest synthesis reaction of elements with a charge
number z +1 at a charge number z of the electrode material. In
particular, this conclusion was confirmed by the synthesis of zinc
using copper electrodes in [6]. The main goal of this work is to
detect rhenium and tantalum, the formation of which is expected
as a result of the interaction of quasineutrons with tungsten ions
extracted during electrode erosion [8].
About the Chemical Composition of the Particles of the Precipitated Powder
The initial chemical composition of the electrodes was determined at several randomly selected locations on the surface of the tungsten electrodes. One of the typical results correspond the measurement uncertainty is 0.01%. We emphasize that neither rhenium nor tantalum was fixed in the composition of the electrode material. After several discharges, the polished surface of the electrodes clearly contained traces of material extraction. Figure 1 shows a fragment of the electrode surface: the light areas correspond to the areas of material extraction, and the dark areas correspond to the original intact surface. After discharges, an inhomogeneous composition is observed on the electrode surface due to the formation of chemical elements (for example, (in wt%) 97.67 W, 0.84 Fe, 0.65 Ag, 0.84 O or (in at.%) 87.84 W, 2.49 Fe, 1.01 Ag, 8.66 O). Without listing other options, we note that neither rhenium nor tantalum was found on the surface of the electrodes. Note that in [2], the appearance of Re, Os, Tm, Au was recorded on the surface of the electrodes. Most likely, their absence on the surface of the electrodes in our experiment is due to the short duration of electrolysis (only a few discharges were carried out) and the rather intense erosion of the electrodes. The main attention was paid to the analysis of the powder obtained after the evaporation of the solution formed in the course of electric discharges in water. Figure 2 shows the appearance of a part of the powder attached to an adhesive tape.
Figure 1: Fragment of the electrode surface after two discharges (magnification 200x).
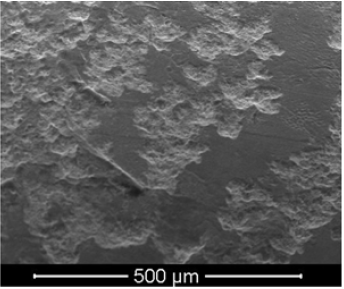
Figure 2: The view of a fragment of powder, fixed on scotch tape, with an increase of 500x.
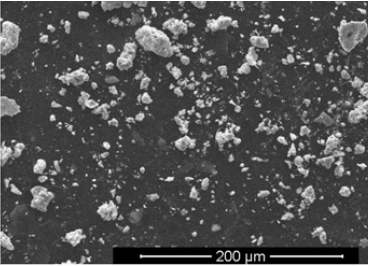
The distribution map of elements shows that rhenium is present in the elemental composition of a number of particles. Table 1 shows the chemical composition of one of the spherical particles with a diameter of ≈1μm. Particles containing tantalum have also been found. Table 2 shows the chemical composition of one of these particles. A characteristic feature of the particle composition is not only the formation of tantalum, but also a low tungsten content with a high niobium content. Particles containing neither rhenium nor tantalum are also observed. The composition of one of these particles is shown in Table 3. Note that a number of other elements are also observed. It is essential that the formation of elements occurred if a gas phase appeared in the cell during the electric discharge. Since the main goal of the work is to confirm the formation of elements adjacent to tungsten in the periodic table, we will not discuss here the reactions leading to the formation of other observed elements. These reactions, especially the formation of niobium, deserve separate consideration.
Table 1: The chemical composition of one of the particles containing rhenium.

Table 2: The chemical composition of one of the particles containing tantalum.
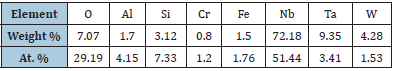
Table 3: The chemical composition of a particle without Re and Ta.

Discussion of Results
First of all, let us indicate the main reactions of rhenium synthesis during the fusion of tungsten nuclei with a proton, escorted by an electron. Recall that tungsten contains four stable isotopes W182 (26.50%), W183 (14.31%), W184 (30.64%), W186 (28.43%). Another isotope W180 (0.12%) has a half-life much longer than the age of the Universe. Hence, it is clear that the synthesis of rhenium, consisting of a mixture of two isotopes: stable Re185 (37.4%) and long-lived Re187 (62.6%) with a half-life T≈4.1⋅1010 years, is quite expected:
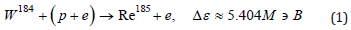
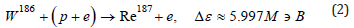
Estimates of the binding energy Δε in exothermic reactions (1) and (2) were obtained using tabular data on atomic masses without taking into account the quasineutron binding energy. Three additional unstable isotopes Re181 (T≈19.9h), Re183 (T≈70d), Re184 (T≈35.4d) immediately after formation can also contribute to the rhenium content, however, β+ - decay or electron capture leads to the stable isotopes W183, W182 and the stable isotope Ta181 (natural tantalum consists of a mixture of a stable isotope and a stable isomer: Ta180m (0.012%), Ta181 (99.988%)). Note that the capture of a quasineutron by the W180 isotope with the formation of an unstable isotope W181 also (after electron capture) leads to Ta181. Reactions
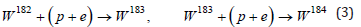
can lead to a change in the fraction of stable isotopes, but not to the nuclei of new elements. On the contrary, the reactions
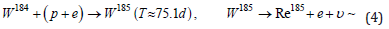
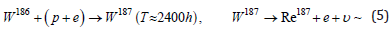
lead to the formation of rhenium.
Assuming reactions (1) and (2) the most probable for the formation of rhenium isotopes, we can assume that the expected ratio of the fractions of rhenium isotopes [f (Re187)/f (Re185)] exp≈ is given (in the “zero approximation”) by the ratio of the corresponding fractions of isotopes tungsten in nature
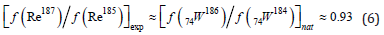
Result (6) sharply differs from the characteristic ratio of rhenium isotopes in nature. The absorption of an electron by tungsten nuclei leads to only one stable isotope of tantalum, Ta-180. Isotopes Ta-182 (T≈114.74d), Ta-183 (T≈5.1d), Ta-184 (T≈8.7h), Ta-186 (T≈10.5m) undergo β- decay, reducing initial isotopes of tungsten W-182, 183, 184, 186. Note that tantalum isotopes can also arise during the capture of a pseudoproton (p+2e) by tungsten nuclei, which, according to [9], is a bound state of a proton with two electrons. For example
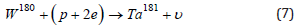
where ν is the symbol of the electron neutrino. The inclusion of one neutrino in the right-hand side suggests that the quasineutron state (p+e), like the neutron, is assigned a zero-lepton charge, while the pseudoproton (p+2e), like an electron, has a unit lepton charge. As a result, in reaction (7), the lepton charge conservation law is formally fulfilled. Along with the synthesis of rhenium during the capture of a proton by the isotopes W184,186, the capture of a proton by the isotopes W184,183 can be accompanied by a reaction with the formation of tantalum and helium
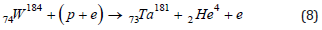
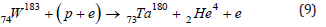
If (8) and (9) are the main reactions of the formation of Ta, then the expected ratio of the fractions of isotopes [f(Ta181)/f(Ta180)] exp will actually be set by the ratio of the corresponding fractions of tungsten isotopes in nature
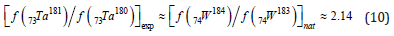
This would be in stark contrast to the proportion of tantalum isotopes in nature
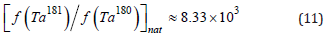
We neglected the contribution of the reaction 180 180
74W + e →Ta ,
as well as (7), since the content of the isotope W180 in nature is low.
In the case of a short electrolysis time, and most importantly, for
short time intervals (compared to T in (3)-(5)) between electrolysis
and mass spectrometry of the solid fraction of electrolysis products,
the neglect of possible reactions (3)-(5) is also justified.
However, it is clear in advance that the absorption processes
of quasineutrons cannot fundamentally affect the difference in
orders when comparing (10) and (11). We emphasize that the
prediction of the expected quantitative ratio of the fractions of
isotopes of the formed elements within the framework of the
concept of quasineutron states was made earlier [4] for the ratio of
the fractions of zinc isotopes, when zinc is synthesized as a result
of electric discharges in water using copper electrodes. In the case
of the formation of rhenium and tantalum, the differences between
the expected (based on experiments on the formation of isotopes)
and those observed in nature are much more dramatic. This can be
seen when comparing data (6) and (7) and especially (10) and (11).
Conclusion
The formation of rhenium and tantalum during the passage of electric current pulses in water in the case of tungsten electrodes supports the concept of quasineutron states. Moreover, in the simplest case of a reaction with the absorption of a proton, it is not difficult to propose an estimate for the ratios of the fractions of the formed isotopes of rhenium and tantalum. A significant difference between these ratios from those observed in nature would not only be an additional confirmation of the artificial origin of rhenium and tantalum but would also directly indicate the mechanism of their occurrence. It seems expedient to check the conclusions about the ratio of isotopic fractions of the formed elements using mass spectrometry.
Acknowledgment
The authors express their gratitude to the Ministry of Science and Higher Education of Russia for their support in the execution of state assignment No. 075-00243-20-01 dated 26.08.2020 within the framework of the theme FEUG-2020-0013 “Environmental aspects of rational nature management”.
References
- Vachaev AV, Ivanov NI (2003) Method of A V Vachaev-N I Ivanov. Interconversion of chemical elements. In: Balakirev VF (Ed), UB RAS, Yekaterinburg, Russia, pp. 28-48.
- Cirillo D, Iorio V (2006) Transmutation of metal at low energy in a confined plasma in water. Proceedings of the 11th International Conference on Condensed Matter Nuclear Science. In: Biberian JP (Ed), World Scientific, London, United Kingdom, pp. 492-504.
- Kashchenko MP, Balakirev VF (2018) A model for intermediate quasi-molecular state and variants of chemical element synthesis. Letters on Materials 8(2): 152-157.
- Kashchenko MP, Kashchenko NM (2021) Formation of massiv electron pairs as a necessary condition for low-temperature nuclear fusion and the existence of a new state of matter. Journal of Micromechanics and Moleсular Physics.
- Santilli RM (2001) Foundations of Hadronic Chemistry. With Applications to New Clean Energies and Fuels. Kluwer Academic Publishers, London, United Kingdom.
- Kashchenko MP, Kashchenko NM (2020) The role of the electronic current component in the formation of a quasi-molecular state leading to the synthesis of elements. Letters on Materials 10(3): 266- 271.
- Kashchenko MP, Kashchenko NM (2019) On the mechanisms of bismuth transmutation in a BiPb melt under the influence of nanosecond electromagnetic pulses. Letters on Materials 9(3): 316-321.
- Kashchenko MP, Balakirev VF, Kashchenko NM, Smirnov MB, Chepelev YL, et al. (2019) Apparent experimental confirmation of pseudoprotons and their application to new clean nuclear energies. International Journal of Applied Physics and Mathematics 9(2): 72-100.
- Kashchenko MP, Balakirev VF, Kashchenko NM, Smirnov MB, Chepelev YL, et al. (2020) The concept of quasineutrons and the synthesis of zinc from the extraction of a part of the material of copper electrodes during electric current discharges in an aqueous solution of NaCl. Letters on Materials 10(4): 486-490.
© 2021 Kashchenko NM. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






