- Submissions

Full Text
Aspects in Mining & Mineral Science
Optimization of Bath Parameters for Electroless Nickel Plating Using Fractional Factorial Design
Shobha R
1Department of Industrial Engineering, MS Ramaiah Institute of Technology, India
2Department of Mechanical Engineering, MS Ramaiah Institute of Technology, India
3Department of Mechanical Engineering, The University of Akron, USA
*Corresponding author: TS Srivatsan, Department of Mechanical Engineering, The University of Akron, Akron, OHIO 44325, USA
Submission: November 15, 2021;Published: December 14, 2021

ISSN 2578-0255Volume8 Issue2
Abstract
Electroless nickel coating is a revolutionary coating process that can be effectively used to cover a spectrum of alloys and composites, each having its own features. Electroless nickel coatings have been primarily utilized for purposes of both corrosion resistance and wear resistance. Additional qualities, such as overall smoothness of the deposit, low friction, acceptable plating rate, and both electrical properties and magnetic properties, makes them an appropriate choice for a wide variety of applications. Characteristics of the electroless nickel coating are essentially determined by both constituents of the electroless solution, and the conditions used during deposition. Bath temperature, nickel source concentration, and pH of the solution are three key deposition parameters. Furthermore, heat treatment does tend to alter microstructure of the coating and thereby exert an influence on hardness, wear resistance and corrosion resistance. In recent years, researchers have published their findings pertinent to an evaluation of electroless nickel coating on the performance of various types of substrates based on hardness, roughness, corrosion resistance, friction, and wear resistance. Several viable ways aimed at solving the challenges specific to parameter optimization have been put forth in the published literature. In this research study, the electroless coating process was performed based on fractional factorial design. The optimal coating parameters were determined using the Analysis of Variance (ANOVA) approach. The findings reveal bath composition can be used to optimize thickness of the coating.
Keywords: Electroless nickel plating; Design of experiments; Analysis of variance; Interaction plots
Introduction
The method of making a nickel alloy by pouring a water-like solution onto the surface and without the use of an electric current is referred to as electroless plating of nickel. Electroplating, on the other hand, relies on direct current as the external source to ensure that the nickel ions in an electrolyte react with the nickel metal at the level of the substrate to form a plating on the nickel metal. Electroless nickel plating is a chemical method that essentially uses chemical reduction to convert the nickel ions in solution to the metal nickel. Sodium hypophosphite is a commonly used reducing agent. Both sodium borohydride and dimethylamine borane are viable alternatives, though they are not used as often as they should be. More than 99 percent of all electroless nickel is made using sodium hypophosphite. Electroless nickel often finds for itself use in a spectrum of industries due in essence to a combination of unique qualities that include the following:
(a) Homogeneity in thickness,
(b) Surface hardness,
(c) Magnetic responsiveness, and
(d) Corrosion resistance.
Despite these notable advantages, not all designers, engineers,
metallurgists (materials engineers), and others involved in the
development of a product are equally qualified to use electroless
nickel [1]. Nevertheless, in recent years this plating technique has
gradually grown both in stature and significance to become wellestablished
as a functional coating in the industries specific to
(i) Food grain manufacturing,
(ii) Electronics,
(iii) Electrical,
(iv) Oil and gas,
(v) Chemical,
(vi) Aerospace, and
(vii) Automotive
Others have noticed and put to effectively use the benefits offered
by electroless plating for a spectrum of applications. Conventional
plating, often known as ‘electroplating’, is a process of reducing the
metal ions to their metallic state and subsequently depositing them
onto the desired location. Electrical energy is often used to power
the cathode. During electroless plating using an aqueous solution,
a metal ion is reduced through a catalytic reaction and forms the
basis for a chemical reduction process containing a reducing agent
that aids in allowing the deposition to occur. Without the need of
electrical energy, the metal can be shaped. A completely different
stabilizer mechanism is often used in electroless nickel plating.
This mechanism is made to be cost-effective [2]. This revolutionary
technology offers the additional advantage of brightening metals
that have a dull surface finish by producing thicknesses of the order
of 100mm without the occurrence of both pitting and microscale
roughness at the fine microscopic level.
Approach
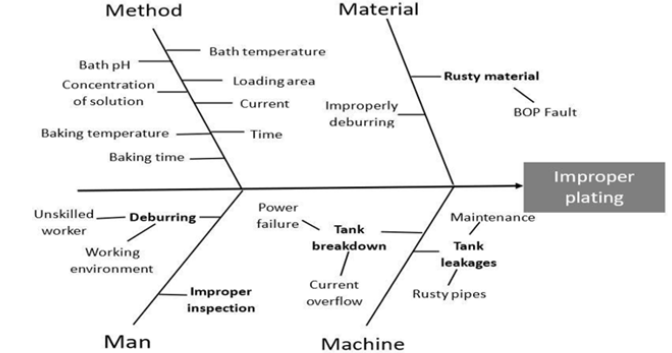
The cause-and-effect diagram
Cause and effect diagram is a tool, which helps to identify the possible causes of a specific problem or a quality characteristic (Figure 1). It graphically illustrates the relationship among the factors identified, which exert an influence on the outcome(s) selected.
Figure 1: The cause-and-effect diagram.
Design of experiments
Based on information available in the published literature [3-
7], it was decided that few experiments would be carried out by
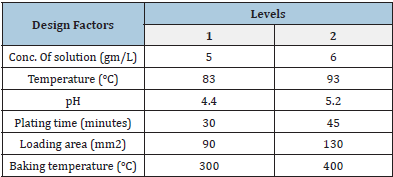
varying the following six factors:
(i) Nickel metal concentration in the solution,
(ii) Bath temperature,
(iii) Solution pH,
(iv) Plating time,
(v) Loading area, and
(vi) Heat treatment temperature.
Based on the prevailing operating situation, the number of
factors was chosen to be both high and low. In this specific study,
a 16 fractional factorial design with two replicates was chosen.
The respective levels are provided in Table 1. There would be 16
runs based on the design chosen for two replicates. The response
variable of interest was thickness of the plating.
Table 1: Factors and their respective levels.
Experimental procedures
The randomization order was determined using the Minitab software [8,9]. The list of randomized orders (indicated by run order) is provided in Table 2. The standard order refers to the Yates design order. The electroless nickel plating was carried out in a 1000ml electroless plating bath. The experiments were carried out in a randomized order, and the test results systematically recorded for the purpose of analysis and interpretation.
Table 2: Plating thickness of the experiment conducted.

Results and Discussion
Thickness of the electroless Nickel plating was systematically determined using the XRF-based coating thickness measurement.
Normal probability plot
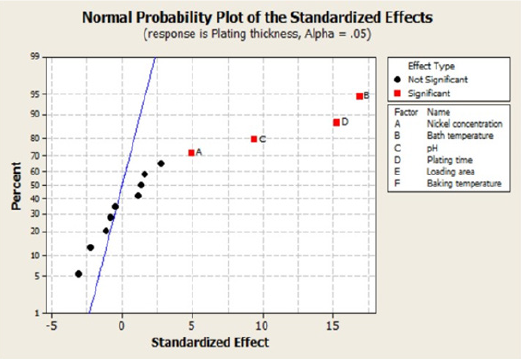
The normal probability plot is as shown in Figure 2. The effects
that are plotted along the line are not significant, whereas the main
significant effects are away from the line and shown by the boxes
placed on the right. The outcomes of this research study arising
from the experiments conducted are the following:
i. Bath temperature,
ii. Plating time,
iii. pH, and
iv. Nickel concentration.
Figure 2: The normal probability plot showing the standardized effects.
Pareto chart
The bars representing factors of bath temperature, plating time and bath pH cross the reference line in this pareto chart, which is 1.456. For the current model, these factors become statistically significant at 0.05 percent.
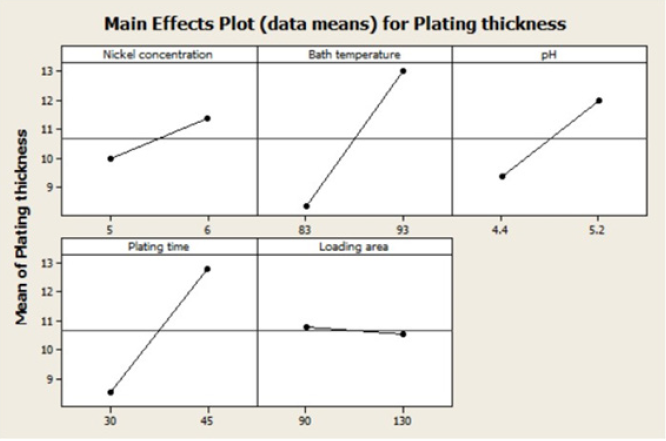
Main effects plot
The influence of nickel concentration, bath temperature, pH and plating time have a positive trend. It is possible to run all the four variables at a chosen high level to optimize thickness of the nickel plating. The loading area was observed to have minimal influence on thickness of the nickel-plating. In fact, its effect was negative thereby indicating that to increase thickness of the nickel plating the loading area should be minimal. Standardized effect plays an important role in the process of plating thickness. Among the six parameters considered; a, b, c and d play an important role. That means nickel concentration, pH, plating time and bath temperature can be safely considered to being both important and essential towards establishing an optimal thickness of the plating (Figures 3 & 4).
Figure 3: The Pareto chart showing the effects.
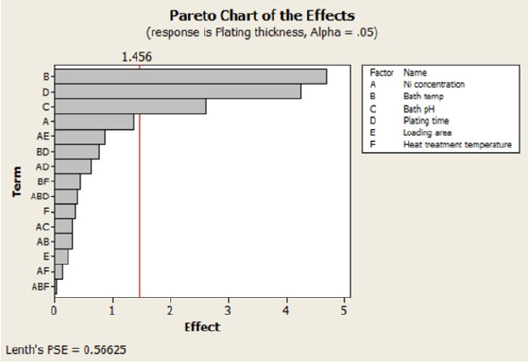
Figure 4: The main effects plot for plating thickness.
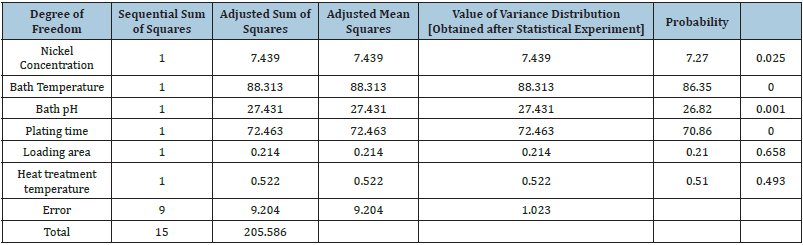
Analysis of variance (ANOVA)
The Analysis of Variance [ANOVA] for thickness of the nickel plating was calculated using MINITAB software and adjusted for the tests [9,10]. The details are tabulated in Table 3. From this ANOVA table for the experiments conducted, the ‘p’ value is low for bath temperature, bath pH and plating time. Hence, the major factors contributing to plating thickness are (i) bath temperature, (ii) bath pH, and (iii) plating time.
Table 3: ANOVA table for the chosen factors.
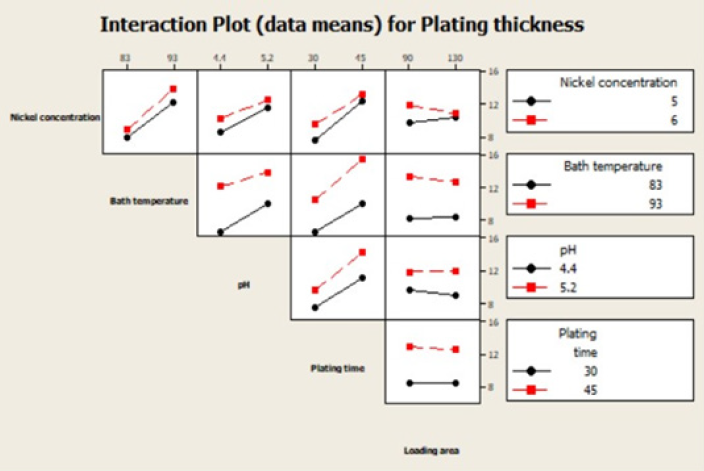
Interaction plot
From an interaction plot for the plating thickness, we observe that all lines are parallel with no interactions occurring between the chosen factors (Table 4). The figure depicts the extent of correlation between two factors especially when the factors are interacting (interdependent). In the graph shown all the factors selected except loading area and nickel concentration are slightly indirect (Figure 5). All other factors do exert or have a direct correlation with thickness of the plating
Table 4: ANOVA table for main factors and interaction effects.

Figure 5: Interaction plot for plating thickness.
Results and Discussion
(a) The current study brings to light the importance of design
of experiment approach in the electroless plating of nickel.
(b) By using fractional factorial design and by varying the
six key bath parameters, the experiments were conducted.
Thickness of the components was determined using a
spectrometer.
(c) The results were carefully analysed using MINITAB
software, to establish the analysis of variance (ANOVA), normal
probability, main effect and the interaction effects.
(d) From the results the main factors that exert an influence
on thickness of the nickel plating are (i) bath temperature, (ii)
bath pH, and (iii) plating time.
(e) In order to obtain a specified thickness of the electroless
nickel plating the corresponding values of the bath parameters
can be obtained from the contour plots.
References
- Krishnan KH, John S, Srinivasan KN, Praveen J, Ganesan M, et al. An overall aspect of electroless Ni-P depositions-A review. Metallurgical and Materials Transactions A 37: 1917-1926.
- Duncan RN (1996) The metallurgical structure of electroless nickel deposits: Effect on coating properties. pp. 68-69.
- Kundu S, Sahoo P, Das SK (2014) Optimization studies on electroless nickel coatings: A review. International Journal of Manufacturing, Materials, and Mechanical Engineering 4(4): 1-25.
- Das SK, Sahoo P (2010) Wear performance optimization of electroless Ni-B coating using Taguchi design of experiments. Tribology in Industry 32(4): 17-27.
- Deepthi YP, Krishna M (2018) Optimization of electroless copper coating parameters on graphite particles using Taguchi and grey relational analysis. Materials Today Proceedings 5(5).
- Omar R, Aboraia MS, Oraby EA (2019) Optimization of the electroless Ni-P coating in glycine bath by Taguchi method. Journal of Engineering Sciences 47(1): 39-47.
- Lelevica A, Walsh FC (2019) Electrodeposition of Ni-P alloy coatings: A review. Surface & Coatings Technology 369: 198-220.
- Das SK, Sahoo P (2009) Optimization of electroless nib coatings based on multiple roughness characteristics. International Conference on Mechanical Engineering pp. 1-6.
- Sarkar S, Baranwal RK, Lamichaney S, De J, Majumdar G (2018) Optimization of electroless Ni-Co-P coating with hardness as response parameter: A computational approach. Journal Tribology 18: 81-96.
- Sarkar S, Baranwal RK, Banerjee S, Prakash A, Mandal R, et al. (2018) Parametric optimization of process parameters on the response of microhardness of electroless Ni-P coating. Journal of Molecular and Engineering Materials 6(1&2): 1-8.
© 2021 Srivatsan TS. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






