- Submissions

Full Text
Aspects in Mining & Mineral Science
Metal Oxide Nanoparticles for Energy Storage and Photocatalytic Applications Towards Environment Benignity
Pavitraa V and Nagarajua G*
Department of Chemistry, Siddaganga Institute of Technology, India
*Corresponding author: Nagarajua G, Energy Materials Research Laboratory, Department of Chemistry, Siddaganga Institute of Technology, Tumakuru-572103, India
Submission: April 02, 2019;Published: December 01, 2021

ISSN 2578-0255Volume8 Issue1
Abstract
The source of energy is the source of human survival and development. The most promising natural source of energy is sunlight. Currently new Metal oxide nanoparticles, which are green, renewable, and alternative to the fossil fuels are exploring due to their wide applications and also key anode materials to replace the graphite in battery technology. Lithium-Ion Batteries (LIBs) have developed rapidly and controlled the market. Besides, Metal oxide semiconductor photo catalysis is a vital process utilizes the solar energy aids for tackling the industrial waste management. This process has been proven to be an efficient technique for the elimination of pollutants from aqueous and gaseous media.
Keywords: Nanoparticles; Anode; Lithium-ion batteries; Photocatalysis
Introduction
With a rapid decline and exhaustion of non-renewable energy sources and unreliable renewable energy sources such as wind, solar, biomass, tide, geothermal. Our challenge is to develop large energy storage materials in order to overcome from the surge of fuels and environmental issues. Therefore, it is essential for modifying recurring renewable resources though incorporating them safely and without problems on the network [1]. Environment pollution can be mitigated by finding battery materials for energy harnessing; besides these materials also acts as good photolytic materials. Photo catalysis is a pollution and residual dyes degradation process in the fields such as medicine, cosmetics, agricultural, electronics, coatings, plastics, textiles, etc. which are not readily biodegradable. Various physical, chemical and biological techniques including ozonization, chlorination and filtration have limitations in energy sources. Therefore, photo catalytic method has developed for alleviating the negative environmental impact of toxic water and pollutants [2]. Metal Oxides (MO) are capable of forming large diversity of compounds by simple synthesis mechanism than carbon, alloy, sulphides, titanium and other organic composites materials. These can be implemented with a large number of structural geometries with electronic structure that displays the role of metallic, semiconductor or insulator nature. In technological aspects, oxides are used in the wide variety of applications [3]. Oxides in nano scale exhibit unique and distinct physical and chemical properties due to their particle size, surface area and density. Particle size will influence in the modification of lattice symmetry and cell parameters. Thermodynamic stability is associated with the low surface free energy; it is achieved by the nano sized metal oxides [4]. Various MO Nanoparticles (NPs) have been investigated extensively as hosts for ion insertion (Lithium, Sodium) for energy storage applications and photocatalytic applications. Structural and electronic properties also drive to modify the band gap of metal oxides to increase the chemical reactivity. Lithium and sodium are in same main group, similar chemical features. Sodium is beneficial in terms of abundance and cost. Due to commercialization perspective Lithium-Ion Batteries (LIBs) are more promising for achieving large scale sustainable portable electrical devices because of its high storage capacity, power density. Negative electrode (anode) is very essential part of LIB’s. Here in this review, we discussed few metal oxide nanoparticles for the negative electrode for LIB’s and for photocatalytic degradation.
Lithium-Ion Batteries (LIBs)
Lithium-Ion Batteries (LIBs) have developed rapidly and controlled the market of battery industry. To explore and improve the power capability and life span of LIBs for storage medium, transition metal oxides (MO, M is Sn, Mo, Ta, Ti and Cu) are very promising anodes and have been extensively studied for electrochemical performance because of high theoretic capacity and improved rate capability [5]. Metal oxides are chosen based on electrochemically active and passive metal in an oxide. Batteries work according to the de-intercalation /intercalation of lithium ions during discharge/charge from cathode and migrate across electrolyte, without altering the crystal structure. Figure 1 explains the schematic representation of LIBs during discharge and charge process. Set up consists of cathode (Li metal), an anode (MO) and electrolyte (1M LiPF6) was dissolved in ethylene carbonate, dimethyl carbonate and diethyl carbonate as conductor in order to get rid of electrolyte diffusion. Electrochemical interface between electrodes and electrolytes takes place during redox mechanism.
Figure 1: Schematic representation of LIBs during discharge and charge process.
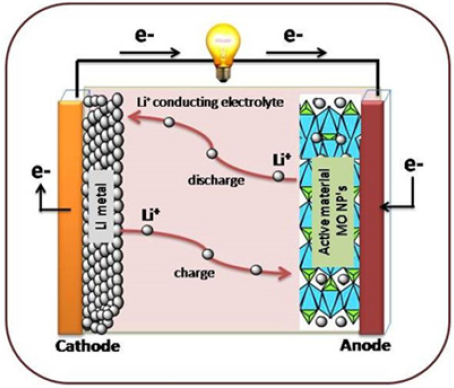
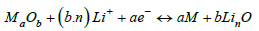
Where M=Transition metal, n=Oxidation state of oxide
MOs are superior battery material exhibits extraordinary
electrochemical behaviour such as high discharge irreversible
capacity. Abrupt initial irreversible capacity induced by Li2O (solid
electrolyte interface) can be effectively reduced; this intends to
decrease the volume expansion. Moreover, helps to accommodate
more lithium ions. Crucial issues such as capacity retention and
stability can be minimized by tuning the size of NPs, different
shapes (flowers, rods, wires, and dumbbells) and dimensions
(3D, 2D and 1D) of structures. Synthesis method also helps for
mass production as well as to eliminate the impurities/defects.
We focus on SnO2, MoO3, Ta2O5, TiO2 and CuO metal oxides; these
forms attractive anode materials with high energy density and
stable capacity retention [6,7]. Park MS et al. [8] fabricated the
self-catalyzed grown one-dimensional SnO2 nanowires by thermal
evaporation method for electrochemical applications. The 1D
nanowire structure provided more reaction sites on the surface
and enhanced the charge transfer process in the electrochemical
reaction. Higher reversible specific capacity of over 300mAhg-1
was obtained up to 50th cycle. In addition, tin particles at the tips of
nanowires contributed to Li+ storage and avoided capacity fading
that is induced by existing metal catalysts. [8]. Zhou J et al. reported
hexagonal MoO3 nanorods for the high-performance lithium-ion
batteries. Hexagonal MoO3 anodes exhibited capacity of 780mAhg-1
after 150 cycles at 150mAg-1 current density. The h-MoO3 nanorods
exhibited high capacity, cycling stability, good rate capability and
less electrochemical impedance due to 1D nanorod morphological
structure [9].
Manukumar KN, et al. [10] synthesized mesoporous high surface
area Ta2O5 nanoparticles via hydrothermal method as an anode
material for lithium-ion battery. Significant reversible capacity of
150mAhg-1 is obtained at C/10 current rate after 50 cycles and also exhibited remarkable hydrogen generation of 563.5μmolg-1h-1 [10].
Ren Y et al. discussed TiO2 (B) nanoparticulate electrodes were
prepared via hydrothermal route to obtain the highest capacity
for the viable commute between lithium ions and the electrodes.
Nanoparticulate titanates exhibited superior volumetric capacity at
all rates compared with the 6 nm anatase material and reported best
high rate (>1000 mAg-1) volumetric capacity [11]. Xiang JY et al. [12]
synthesized the self-assembled CuO hierarchical nanostructures
such as leaf, flower, shuttle, caddice clew and dandelion for LIBs.
In this, they tailored the morphology by adjusting the pH value.
Compared to other structures dandelion and caddice clew like
CuO morphologies exhibited 385mAhg-1 and 400mAhg-1 at 0.1C
and these were maintained stable discharge capacity of about 340
and 374mhg-1 0.5 C after 50 cycles respectively. Excellent discharge
capacities and cyclic performances were due to large surface area,
porosity and lower diffusion length between electrolyte and lithium
ions [12].
Photocatalytic Dye Degradation
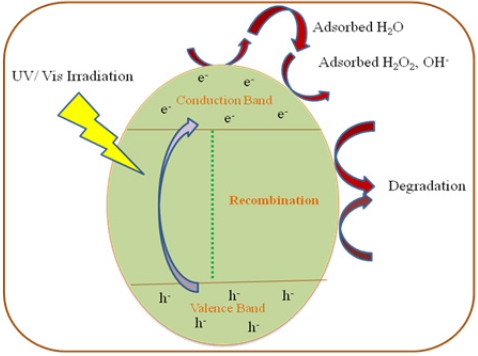
Photocatalytic degradation process uses the nanoscale semiconductor as photocatalyst materials. These catalysts use the lighting power (UV/visible) to produce electron/hole pair are capable of carrying out the oxidation and reduction process on the surface of photocatalyst. It produces reactive oxygen species (hydroxyl and superoxide oxygen radicals). Oxygen species are evolved by the redox process for decomposition of contaminants. Photocatalytic efficiency is strongly depending on the wavelength and power of the light source. The physical properties such as defect density, electronic structure, size, surface area, and surface to volume ratio and chemical nature of photocatalytic species also affects for its efficiency. Figure 2 explains the complete dye degradation procedure under the irradiation of UV/Vis light. Herein, we have discussed few metals oxide photocatalytic nanoparticles which are also energy storage materials for the degradation commonly used dyes such as Methylene Blue and Rhodamine B. Metal oxides are very important photo catalysts due to its rich chemistry with multiple valence states and high thermal and chemicals stability. Metal oxides are semi-conducting materials with band gap of 2-4.0eV, we can enhance the photocatalytic activities by tuning the bandgap [13].
Figure 2: Photocatalytic dye degradation mechanism of dyes.
Elango G et al. [14] were synthesized using C. betacea methanolic extract without introduction of more toxic elements to the environment. SnO2 NPs provided the promising results towards organic applications such as methylene blue dye degradation at 365nm. About 1ml of Methylene Blue was mixed with 0.25mg of SnO2 NP and placed in a UV chamber at 365nm. The degradation spectra were recorded from 400 to 800nm in visible UV spectroscopy. The Surface Plasmon Resonance (SPR) band for the blue methylene dye was degraded at 660nm at 70min [14]. Hu C et al. [15] demonstrated the recyclability of MoO3 nanobelts synthesized thru hydrothermal technique for photocatalytic degradation of Rhodamine B under infrared irradiation. Enhanced results have shown for small concentration of dye at 7.5mg/L catalyst degraded upto 90% after 60 minute [15]. Zhu Y et al. [16] reported the nanosized-Ta2O5 powder photocatalyst using the solgel method. Better photocatalytic performance has been achieved for the decline of gas formaldehyde. Calcination’s temperature and time were played vital role on crystal structure and photocatalytic activities Ta2O5 powder. Nanosyzid-Ta2O5 powder has shown good photovoltaics activity under UV light radiation [16]. Liao DL et al. [17] reported the optimized TiO2 nanoparticles for photocatalytic degradation by different surfactants. Photocatalysts were synthesized by sol-gel method. Various shapes and sizes such as spherical, ellipse, cubic nanoparticles and nanorods were obtained by calibrating with surfactants. Cubic NPs prepared with the use of sodium dodecyl sulfactant had a higher photocatalytic activity than other TiO2 nanoparticles [17]. Li J, et al. [18] prepared spindle CuO particles for photocatalytic degradation under halogen tungsten lamp. CuO particles were very good sensitizer photocatalyst so CuO NP’s degraded the Rhodamine B dye in the presence 0.01M H2O2 under the irradiation of 100W halogen sources. They were very stable and could be recycled without considerable loss of activity. Organic pollutants such as Methyl Orange, Methylene Mlue, Erosin B, and P-Nitrophenol can be photo decomposed in similar conditions. These properties showed practical applications in environmental remediation [18].
Conclusion
Battery technology and photocatalysis are considered as the pure technologies in environment perspective in the present worldwide increasing demand for energy. LIBs are hoping to apply in large quantities energy for storage system in the worldwide usage. The photocatalytic activity is also appreciated tool for many hazardous dyes.
References
- Kang H, Liu Y, Cao K, Zhao Y, Jiao L, et al. (2015) Update on anode materials for Na-ion batteries. Journal of Materials Chemistry A 3(35): 17899-17913.
- Sharma M, Jain T, Singh S, Pandey OP (2012) Photocatalytic degradation of organic dyes under UV-Visible light using capped ZnS nanoparticles. Solar Energy 86(1): 626-633.
- Garcia FM, Rodriguez JA (2007) Metal oxide nanoparticles. BNL-79479-2007-BC.
- Lee SH, Deshpande R, Benhammou D, Parilla PA, Mahan AH, et al. (2009) Metal oxide nanoparticles for advanced energy applications. Thin Solid Films 517: 3591-3595.
- Heidari EK, Kamyabi-Gol A, Sohi MH, Ataie A (2018) Electrode materials for lithium-ion batteries: A review. Journal of Ultrafine Grained and Nanostructured Materials 51(1): 1-12.
- Cao K, Jin T, Li Y, Jiao L (2017) Recent progress on conversion reaction metal oxide anodes for Li ion batteries. RSC Materials Chemistry Frontiers 11(1): 2213-2242.
- Scrosati B, Garche J (2010) Lithium batteries: Status, prospects and future. Journal of Power Sources 195(1): 2419-2430.
- Park MK, Wang GX, Kang YM, Wexler D, Dou SX, et al. (2007) Preparation and electrochemical properties of SnO2 nanowires for application in lithium-ion batteries. Angewandte Chemie International Edition 46(5): 750 -753.
- Zhou J, Lin N, Wang L, Zhang K, Zhu Y, et al. (2015) Synthesis of hexagonal MoO3 nanorods and a study of their electrochemical performance as anode materials for lithium-ion batteries. Journal of Materials Chemistry A 14(3): 7463-7468.
- Manukumar KN, Brij Kishore, Manjunath K, Nagaraju G (2018) Mesoporous Ta2O5 nanoparticles as an anode material for lithium ion battery and an efficient photocatalyst for hydrogen evolution. International Journal of Hydrogen Energy 43(39): 18125-18135.
- Ren Y, Liu Z, Pourpoint F, Armstrong AR, Grey CP, et al. (2012) Nanoparticulate TiO2(B): An anode for lithium-ion batteries. Angewandte Chemie 51(9): 2164-2167.
- Xiang JY, Tu JP, Zhang L, Zhou Y, Wang XL, et al. (2010) Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium-ion batteries. Journal of Power Sources 195(1): 313-319.
- Patil SB, Bhojya NHS, Nagaraju G, Viswanath R, Rashmi SK (2017) Synthesis of visible light active Gd3+-substituted ZnFe2O4 nanoparticles for photocatalytic and antibacterial activities. The European Physical Journal Plus 132: 328.
- Elango G, Roopan SM (2016) Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. Journal of Photochemistry and Photobiology B: Biology 155: 34-38.
- Hu C, Xu M, Zhang J, Zhou Y, Hu B, et al. (2018) Recyclable MoO3 nanobelts for photocatalytic degradation of Rhodamine B by near infrared irradiation. International Journal of Chemical Kinetics 51(1): 3-13.
- Zhu Y, Yu F, Man Y, Tian Q, He Y, et al. (2005) Preparation and performances of nanosized Ta2O5 powder photocatalyst. Journal of Solid-State Chemistry 178(1): 224-229.
- Liao DL, Liao BQ (2007) Shape, size and photocatalytic activity control of TiO2 nanoparticles with surfactants. Journal of Photochemistry and Photobiology A: Chemistry 187(2-3): 363-369.
- Li J, Sun F, Gu K, Wu T, Zhai W, et al. (2011) Preparation of spindly CuO micro-particles for photodegradation of dye pollutants under a halogen tungsten lamp. Applied Catalysis A: General 406(1-2): 51-58.
© 2021 Nagarajua G. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






