- Submissions

Full Text
Aspects in Mining & Mineral Science
Uranium Recovery Focuses
Hamid Kheyrodin*
Semnan University, Iran
*Corresponding author: Hamid Kheyrodin, Assistant professor, Semnan University, Iran
Submission: April 10, 2021;Published: October 07, 2021

ISSN 2578-0255Volume7 Issue4
Abstract
Uranium is a silvery-gray metal that is part of the actinide family. A uranium atom has 92 protons and 92 electrons, six of which are considered capacitance electrons. Because uranium isotopes are unstable, Uranium is an actinide element, and has the highest atomic mass of any naturally occurring element. Uranium is a chemical element with the symbol U and atomic number 92 The uranium ore must be refined to produce the pure, natural metal uranium. Refined uranium contains three different isotopes or nuclear forms. These three isotopes are U -235, U-234 and U-238, all three of which are radioactive elements. There is only a small amount of U-234. Most natural uranium metal is U-238. Which makes up about 3.99% of uranium metal. While U-235 is only about 0.7% of the remaining value. Only uranium-235 with a half-life of about 704 million years has useful properties for nuclear power reactors, atomic bombs and hydrogen. In order to use U-235 in the production of nuclear energy, it must be present in a concentration of more than 0.7%. Nuclear power reactors require at least 5 percent uranium-235. The atomic bomb, on the other hand, requires up to 90% enriched uranium. Therefore, a very complex and expensive method called enrichment is performed to increase the error of uranium-235 and the production of enriched uranium. What remains is depleted uranium, which contains 99.8% uranium-238 and only 0.2% uranium-235. Its density is 5/19 grams per cc. It is approximately 7.1 times the density of lead, 45.2 times the density of iron and 14.2 times the density of copper.
Keywords: Uranium; Metal; Fuel cycle
Introduction
Uranium is an actinide element and has the highest atomic mass of any naturally occurring element. In its refined state, it is a heavy, silvery-white metal that is malleable, ductile, slightly paramagnetic, and very dense, second only to tungsten. Many of today’s uranium applications are summed up in the unique properties of its core. Uranium-235 is the only natural element available that has fissile isotopes, a feature that makes it widely used in nuclear power plants and nuclear weapons [1]. However, because small amounts of it are found in nature, it needs to be increased under a process called uranium enrichment and its concentration for use in nuclear power plants.
Mini Review
The use of uranium compounds by humans has a long history, so that the use of uranium oxide to create yellow color in ceramic glazes dates back to 79 BC. Yellow glass containing one percent uranium oxide, by R.T. Gunter from the University of Oxford was found in an ancient Roman villa on the promontory of Posilipo in the Gulf of Naples, Italy. From the late Middle Ages, uranium ores were gradually mined from the Hasborg silver mines at Yakhimovo in Bohemia (modern-day Czech Republic) and used for dyeing in glass products. In the early nineteenth century, these were the only known uranium mines in the world. The isotope U-235 is important because under certain conditions it can readily be split, yielding a lot of energy. It is therefore said to be ‘fissile’ and we use the expression ‘nuclear fission’. Meanwhile, like all radioactive isotopes, they decay [2]. U-238 decays very slowly, its half-life being about the same as the age of the Earth (4500 million years). This means that it is barely radioactive, less so than many other isotopes in rocks and sand (Figure 1). Existing and well-known processes in uranium enrichment included electromagnetism, gas diffusion, chemical, photochemical, and centrifuge. Each country has chosen its own method according to its position in terms of technology and related industry and access to the necessary facilities, so economic estimation and comparison of different methods seems a little difficult (Figure 2).
Figure 1: Show number uranium electrons, protons and neutrons.
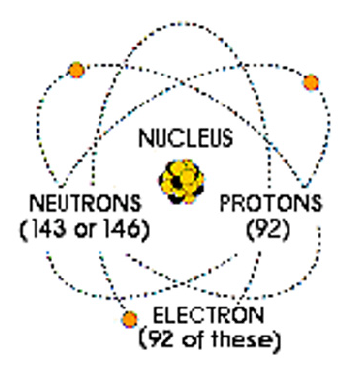
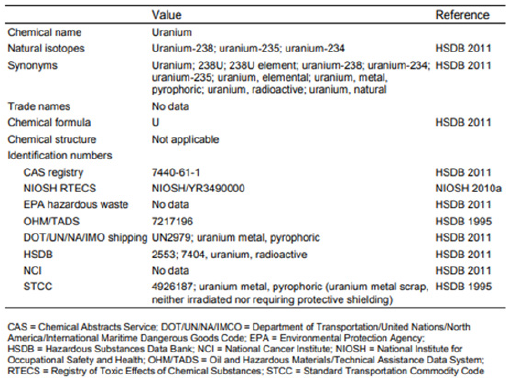
Figure 2: Chemical identity of uranium metal.
Conclusion
There are different methods of uranium recovery as such as: Uranium recovery from industrial effluent by ion exchange. The recovery of uranium from nuclear industrial effluent has been studied using laboratory column and polymeric ion exchange resin. The industrial ..the recovery of uranium from wet. In 2005, seventeen countries produced concentrated uranium oxides: Canada (29.7% of world production), Australia (22.8%), Kazakhstan (10.5%), Russia (8.0%), Namibia (7.5%), Niger (7.4%), Uzbekistan (5.5%), USA (2.5%), Argentina (2.1%), Ukraine (1.9%) and China (1.7%) Kazakhstan continues to increase its production and has probably become the largest uranium producer in the world in 2009 with a projected production of 12,826 tons compared to Canada with 11,100 tons and Australia with 9430 tons. In the late 1960s, UN geologists also discovered significant reserves of uranium and other rare minerals in Somalia. The finding was the largest of its kind, with industry experts estimating more than 25% of the world’s known uranium reserves at 800,000 tonnes. The main condition for uranium enrichment is that the uranium substance is gaseous and, because the only useful substance that can be gaseous by reaction with uranium is fluorine, so in combination with various uranium isotopes it appears as uranium hexafluoride. Due to the uniqueness of the fluorine isotope, in combination with different isotopes of uranium, it adds the same weight to them and thus can be used in the centrifuge; Therefore, the main reason for using the UF6 compound for the uranium enrichment process is the uniqueness of the fluorine isotope.
Acknowledgment
In this article, would like to thank Dr. M. Nasiri the esteemed Dean of the semnan university, for his financial and computer assistance.
References
- Tom Soellner (2010) Uranium: war, energy, and the rock that shaped the world.
- Ian Hore Lacy (2016) Uranium for nuclear power: Resources, mining and transformation to fuel. Woodhead Publishing Series in Energy 47(18): 488.
© 2021 Hamid Kheyrodin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






