- Submissions

Full Text
Aspects in Mining & Mineral Science
Zno/Ag Nanocomposite Thin Films: A Promising Approach for Dye Degradation
Mouna Khiari1,2, Mickaël Gilliot1, Michaël Lejeune2, Florica Lazar1 and Aomar Hadjadj1*
1Matériaux & Ingénierie Mécanique, Université de Reims Champagne-Ardenne, France
2Laboratoire de Physique de la Matière Condensée, Université de Picardie-Jules Verne, France
*Corresponding author: Aomar Hadjadj, Matériaux & Ingénierie Mécanique, Université de Reims Champagne-Ardenne, France
Submission: September 21, 2021;Published: September 27, 2021

ISSN 2578-0255Volume7 Issue3
Opinion
Forecasts for the global textile dyes market would indicate a growth from some US $7 billion in 2019 to more than US $13 billion in 2027, despite stringent environmental regulations [1]. This high consumption of dyes, added to other human activities, will produce large quantities of waste that will end up, most often, in wastewater. Already now, 100 tons of dyes are released annually in effluents by industry [2]. The search for efficient and inexpensive methods for the treatment of these polluted waters is more than topical. Photocatalytic degradation is an effective method to treat this contaminated water and thus reduce environmental pollution. In addition, associating the solar resource in heterogeneous photocatalysis is an additional factor to boost the search for innovative photocatalysts capable of eliminating dyes, pesticides, fertilizers, bacteria, etc., during the treatment of contaminated wastewater. Currently, the most promising semiconductor in this field is ZnO. This wide band gap semiconductor is known for its chemical stability, its non-toxicity, its interesting optoelectronic properties, and the relative ease to deposit it in thin films. Figure 1a shows the growth in the number of publications devoted to the use of ZnO in dye degradation. These publications mainly address the following dyes: Methylene Blue (39%), Methyl Orange (31%) and Rhodamine B (23%). Although Indigo Carmine is the most used for dyeing fibers (like jeans) and despite its harmful effects (toxic and non-biodegradable, its molecule is very difficult to degrade), the share of publications devoted to this dye does not exceed 6%.
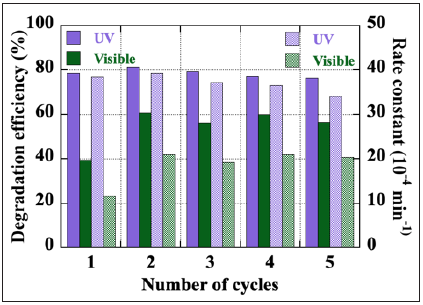
However, with its wide band gap at 3.4eV, ZnO only absorbs the UV part of the solar spectrum which represents only a few percent. The incorporation of silver nanoparticles in the ZnO matrix is a solution to improve the absorption of the nanocomposite in the visible range to allow the use of this inexhaustible natural resource that is the sun. Figure 1b shows the number of papers dealing with ZnO/Ag nanocomposite and dye degradation over the last decade (15% to 20% of papers in Figure 1a). The beneficial contribution of silver to the photocatalytic properties of ZnO, widely reported, concern the narrowing of its band gap and the improvement of its absorption in the visible range, the plasmonic effects of this noble metal in the form of Nanoparticles (NPs), the separation of charges due to the Ag-ZnO Schottky barrier, etc. Among the various techniques used to deposit ZnO thin films, sol-gel processing coupled with spin-coating has many advantages: relative simplicity, low cost, possibility of deposition on large surfaces and control of the morphology of the deposits. In addition, it allows to incorporate directly Ag NPs in the ZnO precursor solution. Figure 2a summarizes the solgel coupled spin coating process for deposition of ZnO/Ag(NPs) nanocomposite thin films [3]. Ag NPs with a spherical shape and an average size of about 100nm are uniformly incorporated on the surface of the nanogranular ZnO thin film (Figure 2b). Such ZnO/Ag(NPs) nanocomposite thin film photocatalyst allows easy separation from the treated water and its recovery for reuse, which is more complicated in the case of photocatalysts in nanopowder form. This quality is a clear advantage for industrial applications. The photocatalytic performance of the ZnO/Ag(NPs) composite in the degradation of a Carmine Indigo solution is displayed in Figure 3. The kinetics of degradation depends, among other things, on the intensity of the illumination used. Although the photocatalytic performance remains higher in the UV, Ag NPs have significantly improved it in the visible range. The ZnO/Ag(NPs) nanocomposite thin film can be used for a longer period. It keeps a stable and efficient photocatalytic performance at least over five photocatalytic cycles of 7 hours and no visible deterioration on the surface of the catalyst can be reported. This work is a contribution to the research of non-toxic thin film photocatalysts that can be used under solar radiation and easily reused. The presence of metallic NPs uniformly distributed on the surface of the 100nm thick photocatalyst and in direct contact with the dye solution is its most promising asset.
Figure 1: Growth in the number of publications on dye degradation by ZnO (a) and ZnO/Ag nanocomposite (b) over the last decade (source Scopus).

Figure 2: (a) Sol-gel coupled spin coating deposition process of The ZnO/Ag(NPs) nanocomposite thin films deposition process. (b) Scanning electron microscopy image of ZnO/Ag(NPs) nanocomposite thin films with 10% content of Ag NPs.
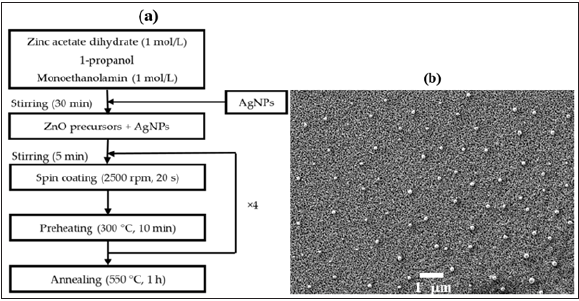
Figure 3: Degradation efficiency and rate constant of a ZnO/Ag(NPs) nanocomposite thin film over 5 cycles of 7h of degradation of the Indigo Carmine solution, under visible- and UV-light illumination.
Acknowledgement
The authors thank the Grand-Est Region (France) and the Hauts-de-France Region (France) for co-funding this study.
References
- (2020) Global Textile Dyes Market Research Report 2018-2020, 2024 & 2027.
- Fan J, Chen D, Li N, Xu Q, Li H, et al. (2018) Adsorption and biodegradation of dye in wastewater with Fe3O4@MIL-100 (Fe) core–shell bio-nanocomposites. Chemosphere 191: 315-323.
- Khiari M, Gilliot M, Lejeune F, Lazar A, Hadjadj A (2021) Effects of Ag nanoparticles on zinc oxide photocatalytic performance. Coatings 11(4): 400.
© 2021 Aomar Hadjadj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






