- Submissions

Full Text
Aspects in Mining & Mineral Science
Graphene Oxides/Porous Silicon Heterostructure for CO Sensors
Dzhafarov TD*, Bayramov AH, Ragimov SX, Bagiyev EA, Nasibov IA and Karimova AM
Institute of Physics, Azerbaijan National Academy of Sciences, Azerbaijan
*Corresponding author: T D Dzhafarov, Institute of Physics, Azerbaijan National Academy of Sciences, Azerbaijan
Submission: April 28, 2021;Published: May 18, 2021

ISSN 2578-0255Volume6 Issue4
Abstract
Graphene oxide is compound made up of carbon, hydrogen and oxygen molecules and the material (semiconductor or insulator) depend on the degree of oxidation and their electronic properties can be tuned in large scope. The current-voltage (I-V) characteristics of n-Graphene oxide/p-Porous silicon (GO/PS) structures in the normal air and CO atmosphere were investigated. The generation of an open-circuit voltage at CO atmosphere for GO/PS structure at room temperature was observed. The sensitivity of GO/PS structures to CO molecules can be used as CO sensors. The mechanism of generation of electricity in GO/PS structure can be related to chemical interaction of CO composition, couples of water and creation of electrons in H2 molecules.
Keywords: Nano-Graphene oxide; Porous silicon; CO gas; Graphene oxide/Porous silicon sensor
Introduction
Investigations and commercial interest of graphene oxide, porous silicon and another nano materials are very intense in the recent year due its potential applications of different types of gas sensors. Gas sensors were divided on next main categories : resistive, capacitance, solid electrolyte, infrared, biomedical and other [1-4]. Graphene oxide contains large number of oxygen-containing functional groups in the chemical structure, so that GO is a three-dimensional structure (a hexagonal lattice) containing both sp2 and sp3. In the tradition structural model, hyraxes, and epoxy (C-O-C) groups are predominantly present in the basal plane of GO, and carbonyl (C=O) and carboxyl groups appear on the edge of GO sheets. Because of the presence of these functional groups, GO is the insulator or semiconductor material. Porous silicon has an extremely large surface-to-volume ratio (up to 800m2/cm3) and high chemical activity of the surface [4]. These properties enable PS to effectively react with gases and porous silicon layers are therefore considered as very promising for sensor applications. The gas-stimulated modification of the electrical or optical properties of PS is generally associated with changes in the conductivity or dielectric constant due the adsorption of gas molecules inside the pores. This provides a base for high-sensitivity and low-cost gas sensors, which are needed in numerous environmental, biomedical and food applications. Some of data about properties of Au (Ag or Cu)-Porous silicon sensors were presentation in [5,6]. In this paper, we fabricate the gas sensor based on a Graphene oxide/Porous silicon structure and show that this device is an effective sensor toward CO gas ambient. The resistance of GO/PS heterostructure changes under action CO gas and in further realizes through porous silicon.
Results and Discussion
We prepared for Graphene oxide of the solution using Modified Hummers method from NaNO3, H2SO4, KMnO, HCl, H2O reactions [7]. Then, the solution was used for electrophoretic deposition of films Graphene oxide on silicon or porous silicon (at U=30V and 4.5mg/ml) at room temperature. Porous silicon with a thickness of 10-20µm were prepared by anodic etching on one surface of (111) oriented p-type silicon wafers with a thickness of 350µm by anodic etching in hydrofluoric-ethanol solution [8]. X-Ray Diffraction (XRD) analyses, Raman spectra, Photo Luminescence (PL) and ellipsometry measurements of the GO films were used in this investigation. The gas-stimulated open-circuit voltage (V) and short-circuit current density (J) were measured by Thurlby-1503 digital multimeter. The concentration of CO was measured using a BW Defender Multi-Gas Detector. All of the measurements were performed at room temperature. X-Ray diffraction pattern and Raman scattering spectrum of Graphene oxide films were corroborate to FCC lattice [9]. The band gap of the GO film found from spectroscopic ellipsometry data and photoluminescence spectrum is Eg=2.37eV and the average thicknesses of films is d=121nm. The current-voltage characteristics of GO/PS heterostructure (RD) in normal air ambient (300K, 45% relative humidity) and under CO gas influence showed in good rectifying properties.
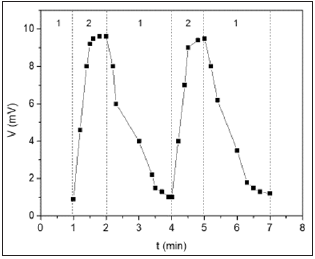
The CO concentration–voltaic effect, the generation of the voltage between the contacts to Graphene oxide nanofilm and porous silicon acting CO-molecules for Graphene oxide/Porous silicon structure was observed at room temperature. Figure 1 illustrates the dependence of the voltage generated in the GO/PG sensor on the CO concentration. It is seen that the voltage approximately linear increases from 1 to 11mV at CO-concentration changes of 250-3500ppm. The sensitivity of open-circuit voltage and short-circuit current to CO molecules (about 6mV/ppm) show of use of GO/PS structure as gas sensor at 300K. Figure 2 show the response-recovery behavior of the voltage for GO/PS sensor on the successive cycles placing in room air atmosphere and in CO ambient (350ppm). It can be seen that the response time is about 60-70s. Concerning the mechanism for the sensitivity of the GO/PS sensor to CO molecules, one can suppose that at first stage, the molecules from the humid air with the CO, resulting in the formation of hydrogen, according to the electrode reaction [1,8]
Figure 1: The voltage response of GO/PS sensor exposed to CO gas concentration.
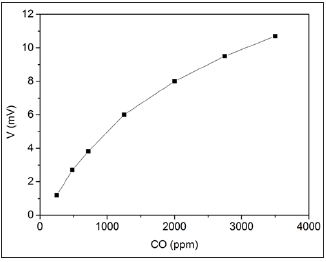
Figure 2: The voltage response of GO/PS sensor as a function of storage time on the successive placing in air (1) and CO gas (2) of 350ppm.
CO+H2O→CO2+H2 (1)
H2→2H+2e- (2)
After the reaction described by eq.(1), the formation of electricity proceeds in accordance with eq.(2). The formation of hydrogen, according to reaction (2) is creating of electrons in Graphene oxide film of the GO/PS sensor a produce of voltage.
Conclusion
In this study, fabrication, and characterization of GO/PS structures with the Porous silicon membrane and Graphene oxide catalyst has been presented. The performance of the GO/PS structures were measured at room temperature by supplying CO gas atmosphere. The test results confirm that the sensor generated the open-circuit voltage of 11mV of CO gas atmosphere. To suggest itself, the operational principle of such Graphene oxide is based on changes in conductivity due to of CO-adsorbs and appear of the donors.
References
- Kohl D (2001) Function and applications of gas sensors. J Phys D: Appl Phys 34: R125-R149.
- Frantzer G, Franzer A, Sanders D, Simon U, Maier WF (2006) Wet chemical synthesis and screeking of thick, porous oxide film for resistive gas sensing applications. Sensors 6: 1568-1586.
- Shuk P, Murphy P (2005) 1st International Conference on Sensing Technology. Palmerston North, New Zealand.
- Dzhafarov TD, Bayramov A (2017) Porous silicon and solar cells. Handbook of Porous Silicon (Ed. Leigh Canham), Second Education. Springer International Publishing AG, Germany.
- Dzhafarov TD, Yuksel SA, Lus CO, Caliskan M, Yesilkaya SS (2010) Au/Porous silicon-based sodium borohydride fuel cells. Int J Energy Res 34(15): 1386-1392.
- Dzhafarov TD, Yuksel SA (2009) Nano-porous silicon for gas sensors and fuel cell applications. J Gafqaz University 25: 20-35.
- Shahriary L, Athawale AA (2014) Graphene oxide synthesized by modified hummers approach. Inter Journal of Renewably Energy Environmental 2(1).
- Dzhafarov TD, Yuksel SA, Lus CO (2008) Porous silicon-based gas sensors and miniature hydrogen cells. Jap Jour Appl Phys 47(10): 8027-8204.
- Gascho JLS, Costa SF, Recco AAC, Pezzin SH (2019) Graphene oxide films obtained by vacuum filtration: X-ray diffraction evidence of crystalline reorganization. Journal of Nanomaterials.
© 2021 Dzhafarov TD. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






