- Submissions

Full Text
Aspects in Mining & Mineral Science
Applications of Chlorination of Copper: Separation, Deposition, and Thermochemical Cycle
Qingchun Yu*, Yong Deng and Xiumin Chen
State Key Laboratory of Complex Non-ferrous Metal Resources Clear Utilization, Faculty of Metallurgy and Energy, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
*Corresponding author: Qingchun Yu, State Key Laboratory of Complex Non-ferrous Metal Resources Clear Utilization, Faculty of Metallurgy and Energy, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
Submission: April13, 2020;Published: November 20, 2020

ISSN 2578-0255Volume5 Issue5
Abstract
Chlorination of copper has distinctive physical and chemical properties, which makes it applicable in fields of mineral processing, material synthesis, and energy production. Chlorination technique for simultaneously extracting Ni and Cu from mixed oxide-sulfide copper‑nickel ore or selective chlorination for the removal of copper from copper slag was developed. Copper film is a promising material which can be produced through the “in situ” chlorination of copper by chlorine and copper deposition via reduction of Cu3Cl3 by hydrogen. The Cu-Cl thermochemical cycle for hydrogen production has significant potential because it does not generate greenhouse gas and requires lower energy consumption.
Keywords: Chlorination; Copper; Extraction; Film; Cu-Cl cycle
Introduction
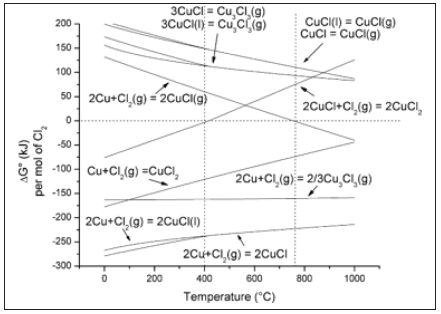
Various studies have been made on the chlorination of copper. Products of chlorination of copper include CuCl, Cu3Cl3, and CuCl2. Possible chemical reactions of standard Gibbs free energy versus temperature are shown in Figure 1. It is found that CuCl is the most likely product to be obtained for the reaction of copper with chlorine, whether in solid, liquid or vapor state. According to the △G° value, formation of gaseous Cu3Cl3 from metallic copper is expected for all temperatures [1]. Formation of CuCl2 from metallic copper cannot be excluded since it also has a negative value of △G°. The reaction of CuCl with Cl2 to give CuCl2 is feasible for temperatures below 400 ℃, at higher temperatures the decomposition of CuCl2 is predictable because the △G° of this reaction is negative. The direct combination of the elements is the most common method of production. However, Remeika & Battlogg [2] studied the synthesis of CuCl from Cl2 and CCl4. They found that very pure and stoichiometric CuCl is produced by the reaction of copper with CCl4. Thermogravimetric study [3] showed that the chlorination of metallic copper proceeded by forming CuCl, followed by further chlorination of a part of CuCl to CuCl2 above its melting point, but the amount of CuCl2 formed was limited. The distinctive physical and chemical properties of chlorination of copper make it applicable in fields of mineral processing, material synthesis, and energy production.
Figure 1: Standard Gibbs free energy versus temperature for the possible chlorination reaction [1].
Mineral Processing
Chlorination roasting presents advantages for processing low-grade polymetallic complex ore. The metal chlorides formed by roasting has low melting point and high volatility and can be separated according to their boiling points and vapor pressure values. Chlorination roasting reagents can be classified into gaseous chlorinating agents, and solid chlorinating agents, such as Cl2, HCl, CaCl2, NaCl, MgCl2 and AlCl3. Solid chlorinating agents are preferred to gaseous ones in industrial production owing to their low price, availability, low equipment requirements and simple operation. The chlorination reactions of copper or copper-containing mixtures were studied for the development of separation methods in extractive metallurgy for the recycling of metals from ores and waste slag. Because of the depletion of the high grade nickel sulfide ore, mixed oxide-sulfide copper‑nickel ore, which accounts for approximately two-thirds of the total nickel reserves of this mine has become the main raw material for nickel products. The low-grade nickel sulfide ore contains a lot of serpentine and talc. In the conventional pyrometallurgical process (flash or electric smelting), the concentrate with high content of MgO will cause the problems of furnace nodulation, large viscosity to slag, difficulties in the separation between matte and slag, low recovery of valuable metals, and etc. A low-temperature chlorinating roasting-water leaching process to achieve the extraction of valuable metals selectively. Corresponding reactions are as follows [4]:
AlCl3+3/4O2(g)=1/2Al2O3+3/2Cl2(g)
CuFeS2+2Cl2(g)=CuCl2+FeCl2+2S
Fe4.5Ni4.5S8+9Cl2(g)=4.5FeCl2+4.5NiCl2+8S
The copper slag from the pyrometallurgical production of copper depending on its origin, contains about 35-45% wt% of iron that can be recovered, if treated suitably. Carbothermic reduction of the waste slag therefore inevitably results in the co-reduction of large amounts of iron together with copper. Higher copper content in the pig iron causes the brittleness and makes the iron worthless. Selective chlorination for the extraction of copper and the upgrading of lean minerals can be achieved by controlling reaction conditions such as the temperature and/or the amount of the chlorinating agent. The resulting copper removal rate of 84.34% is obtained under the optimum conditions [5]. Pickles [6] conducted a study about thermodynamic analysis of selective chlorination of the arc furnace dust. He found that copper could be chlorinated effectively as gaseous chlorides, but a small amount of iron oxides also could be converted to gaseous iron chlorides during the process. By adding some oxygen, the stability of iron oxide was promoted, which went against the conversion of iron oxide to gaseous iron chloride.
Material Synthesis
Copper film is considered as a promising alternative to replace Al and its alloys because of its enhanced resistance to electromigration and low resistivity. Chemical Vapor Deposition (CVD) offers advantages such as its ability to involve the substrate surface in the deposition reaction, leading to a conformal blanket or selective metal growth even on substrates with complex shape [7]. Usually volatile Cu I and Cu Ⅱ organometallic precursors have been used in the CVD process, but these precursors tend to be unstable in classical industrial CVD reactor. Moreover, the maximum growth rate is quite low. A potentially interesting alternative might consist in using copper chloride as copper source in a low pressure CVD reactor. Cu3Cl3 is the only gaseous precursor and will deposit on the substrate as pure CuCl. An excess of hydrogen is needed for the copper chloride reduction, and the overall chemical reaction leading to the formation of copper can be written:
2CuCl+H2(g)↔2Cu+2HC1(g)
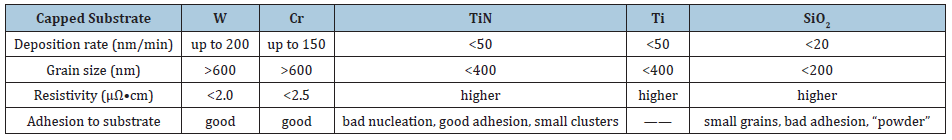
Results showed that the deposition rate depends strongly on the substrate temperature and nature. Pure copper films have been obtained in the intermediate substrate temperature range of 200 ℃ - 430 ℃. (Table 1) summarizes some characteristics of the copper films obtained by chlorination and reduction on different surfaces. It shows that the bad nucleation of copper on TiN and Ti substrate could be attributed to the etching of these surfaces by an excess of chlorine and/or of HCl. On SiO2 substrate, very small grains have been deposited producing a discontinuous layer with a very bad adhesion. The nucleation of copper on this surface is poor. The best deposition rate (200nm/min) that has been obtained so far is on tungsten covered bare silicon substrate [8]. The films deposited on tungsten were found to be polycrystalline. The average thickness of the film is about 1.2μm.
Table 1: Characteristics of copper layers deposited on different substrates [8].
Energy Production
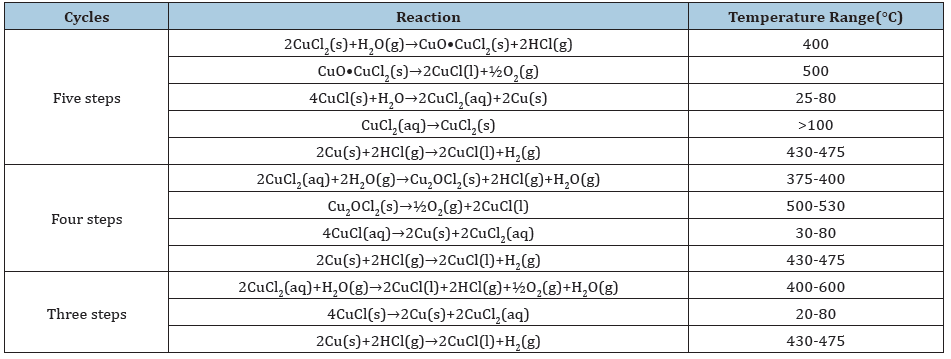
Hydrogen economy would become significant in the next several decades since hydrogen will likely be used in power generation, transportation, and industrial processes. Hydrogen production is a key component of the hydrogen economy. Many methods and technologies currently exist for producing hydrogen. The predominant industrial process is steam methane reforming, which uses methane to produce hydrogen, but releases greenhouse gases as a byproduct. In contrast, thermochemical water splitting cycles are cleaner and potentially coupled to different types of energy sources. They can be linked with nuclear or other thermal power plants to utilize waste heat emitted from the plants. The high temperature waste heat can improve energy efficiency of thermochemical cycles. The copper-chlorine (Cu-Cl) thermochemical cycle is a promising process due to lower operating temperature from 430 ℃ to 475 ℃ and potentially lower cost materials. The process involves a series of closed-loop chemical reactions that does not contribute to any greenhouse gas emissions to environment. Various design schemes for Cu-Cl thermochemical cycles based on several steps of three to five are analyzed and compared shown in Table 2. The five-step Cu-Cl cycle is the first and main design schema that originally developed. All the other new configurations are obtained combining one or more steps in the five-step Cu-Cl cycle. The four-step Cu-Cl cycle is hydrolysis, decomposition (oxygen production), electrolysis (hydrogen production) and water separation by drying or crystallization [9]. The three-step Cu-Cl cycle combines the steps of drying and hydrolysis to carry out aqueous cupric chloride as the feed of the hydrolysis reactor. With the decrease of the number Cu-Cl cycles, kinetic conditions of chemical reactions are excellent because the homogenous mixing and handling of a liquid or gas is usually easier than a solid. However, some disadvantages may appear, such as higher heat grade and intensity requirements, and decrease of the desirable products yield. These disadvantages are more obvious especially in the three-step Cu-Cl cycles [10]. Higher heat grade and intensity can make the design of reactor difficult from a practical engineering perspective.
Table 2: Comparision of Cu-Cl cycles with their corresponding reactions [9,10].
Conclusion
The chlorination of copper proceeded by forming CuCl, Cu3Cl3, and CuCl2 at a moderate temperature makes it applicable in many fields. Chlorination technology is proved to be an efficient method for the extraction of valuable metals and the upgrading of lean ores or waste slags. Copper film can be obtained through the “in situ” chlorination of copper by chlorine and copper deposition via reduction of Cu3Cl3 by hydrogen. The growth rate is acceptable in industrial scale. Thermochemical water splitting is an economical and sustainable production of hydrogen. With decrease of the number of steps it will be difficult for Cu-Cl cycle to separate product particles. With the continuous research, it is believed that the chlorination of copper will be applied to more fields in future.
References
- De Micco G, Bohe AE, Pasquevich DM (2007) A thermogravimetric study of copper chlorination. Journal of Alloys and Compounds 437(1-2): 351-359.
- Remeika JP, Battlogg B (1980) Synthesis of CuCl. Materials Research Bulletin 15(8): 1179-1182.
- Tit Manyaka R, Iwasaki I (1976) Chlorination behaviors of complex iron, copper and nickel sulfides. Trans Soc Mining Eng AIME 260: 282-288.
- Cui F, Mu W, Zhai Y, Guo X (2020) The selective chlorination of nickel and copper from low-grade nickel copper sulfide-oxide ore: Mechanism and kinetics. Separation and Purification Technology 239: 116577.
- Li L, Wang H, Hu J (2015) Smelting chlorination method applied to removal of copper from copper slags. Journal of Central South University 22: 59-65.
- Pickles CA (2009) Thermodynamic analysis of the selective chlorination of electric arc furnace dust. Journal of Hazardous Materials 166(2-3): 1030-1042.
- Bourhila N, Thomas N, Palleau J, Torres J, Bernard C, et al. (1995) Thermodynamic and experimental study of Cu-LPCVD by reduction of copper chloride. Applied Surface Science 91(1-4): 175-181.
- Bourhila N, Torresb J, Palleau J, Bernardc C, Madar R (1997) Copper LPCVD for advanced technology. Microelectronic Engineering 33(1-4): 25-30.
- Mehmet FO, Ibrahim D, Marc AR (2012) Efficiency comparison of various design schemes for copper-chlorine (Cu-Cl) hydrogen production processes using Aspen Plus software. Energy Conversion and Management 63: 70-86.
- Wang ZL, Naterer GF, Gabriel KS, Gravelsins R, Daggupati VN (2009) Comparison of different copper-chlorine thermochemical cycles for hydrogen production. Int J Hydrogen Energy 34(8): 3267-3276.
© 2020 Qingchun Yu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






