- Submissions

Full Text
Aspects in Mining & Mineral Science
Thermodynamics of the Ternary Water-Salt Systems
Allakhverdov GR* and Zhdanovich OA
State Scientific-Research Institute of Chemical Reagents and High Purity Chemical Substances– National Research Centre, Kurchatov’s Institute, Russia
*Corresponding author: Allakhverdov GR, State Scientific-Research Institute of Chemical Reagents and High Purity Chemical Substances- National Research Centre, Kurchatov’s Institute, Moscow, Russia
Submission: November 08, 2019;Published: December 10, 2019

ISSN 2578-0255Volume4 Issue3
Abstract
The article present methods for calculation the thermodynamics characteristics of ternary water-salt solutions based on data for binary solutions of the components that form a mixed solution.
Keywords: Osmotic coefficient; Isopiestic solution; Activity of component
Introduction
The calculation of the characteristics of mixed solution based on the properties of binary solutions is one of the central problems of the theory of solutions. The McKay-Perring equation [1] shows that the calculation of the activity coefficient of the components of mixed electrolyte solutions can be carried out by comparing the experimental data for mixed solutions with the data for binary solutions under the condition of isopiestic equilibrium of all solutions. This condition opens a window for other methods for calculation the properties of mixed solutions based on data tor binary solutions of the individual components of ternary system.
Theory
Suppose that two binary solutions of different electrolytes having the equal vapor pressure over a solution, when mixed, form a solution with the same vapor pressure. If in a mixed solution the concentration of one electrolyte x1 and another x2, then the following relation should hold dx1/dx2=c where C= constant, since the solvent activity remain unchanged. Here x is the comparative concentration
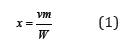
where 𝜈 is the stoichiometric coefficient of the electrolyte, m is molality, W is the number of moles solvent per 1kg (for water W= 55.51). The molar fraction of the components of the mixed solution can be defined as
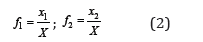
where X = x1 + x2 , and further determine C as
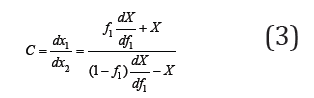
Separating variables and integrating Eq. (3), we obtain
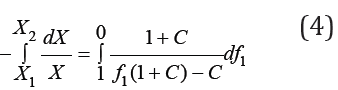
Hence, we have

where X1 and X2 are the concentrations of isopiestic binary solutions. Integrating Eq. (5) taking into account the boundary conditions, we obtain [2]
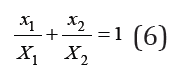
or, using Eq. (2)
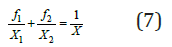
Eq. (6), using Eq. (1), can be represented as
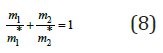
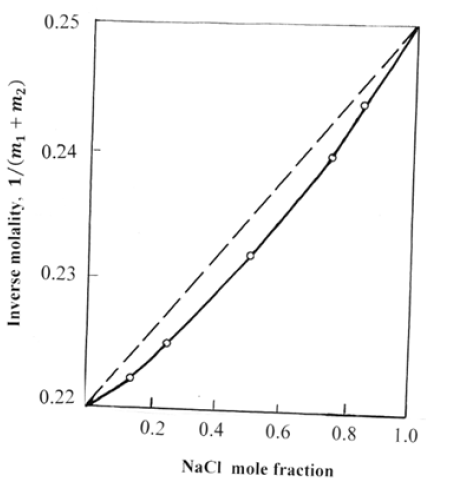
where mi is the molality of the components in mixed solution and mi* is the molality of the components in binary isopiestic solutions. In this form Eq. (8) reflects the empirical rule by Zdanovsky [2], which shows a linear dependence of the inverse molality on the composition of the solution (Figure 1).
Figure 1: The composition of a mixed solution in the system NaCl-KCl-H2O at aw=0.851 (T=298K). The dotted line according to Eq. (8).
At constant pressure and temperature, the Gibbs-Duhem equation for a ternary water-salt system can be represented as
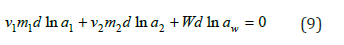
where aw is the solvent activity and ai is the activity of the component in the mixed solution, for example,
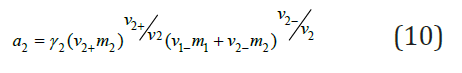
where v2 v2+ v2− and γ2 is activity coefficient in ternary solution. Substitution Eq. (10) into (9) and using Eq. (1), we have
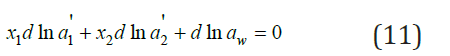
where 'i a = xiγ i . Combining Eq. (7) and (11) we obtain
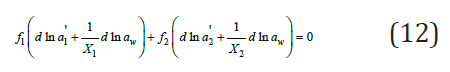
On the other hand, for each binary solution it is possible to write
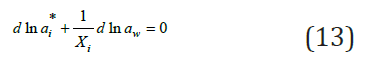
where ai* is the activity of the components in isopiestic binary solution. Combining Eq. (12) and (13) we obtain
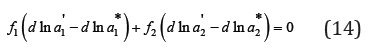
The solution of Eq. (12) can be represented as
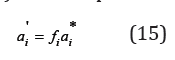
Revealing the activity of the components in the binary isopiestic solutions: ai = Xiγ i , Eq. (15) can be transformed to the form
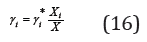
or, moving on to molality
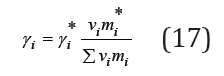
where γi* are activity coefficients in the binary isopiestic solution.
The McKay-Perring equation can be expressed in integral form as
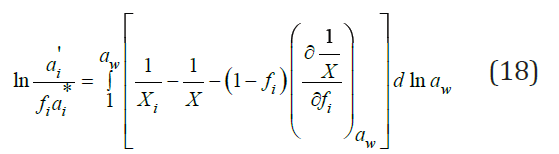
Substitution here Eq. (7) turns the integral expression into zero and Eq. (18) turns into Eq. (15). Thus, Eq. (15) is an approximate solution to Eq. (18). A more rigorous solution can be obtained using equation
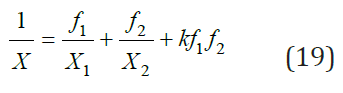
where k is a constant coefficient. Then, according to [2], integrating Eq. (18) we have
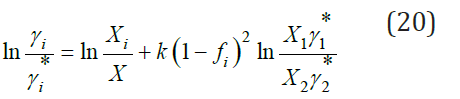
Discussion
Eq. (20) described very well the properties of ternary systems, but the coefficient k can only be determined experimentally. However, for most systems this coefficient is quite small, therefore, below we will consider the practical application of Eq. (7) and the associated Eq. (15-17), which allow us to calculate the activity of the components without involving experimental data for ternary systems. For two binary isopiestic solutions, the equality must be satisfied
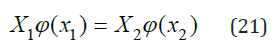
where 𝜑 is the osmotic coefficient. If form of function 𝜑 (x) is known, then for a given concentration of one of the solutions, the concentration of other solution can be calculated. Function 𝜑(x), according to [3], can be represented as
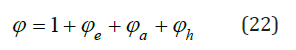
where φe, φa, φh are bound up with electrostatic interaction, association and hydration ions, respectively. Further, using the known ratio
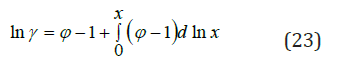
the electrolyte activity coefficient can be defined as
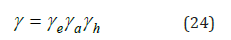
where γe, γa, γh are corresponding constituents:
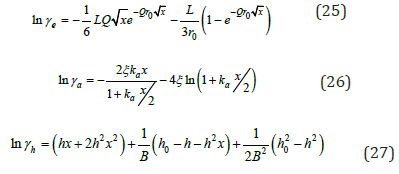
where the first term of each equation is corresponding to φi.
Here r0 is ionic radius, ka is ion association constant, h = h0 exp(−Bx)
, where h0 is hydration number in infinitely dilute solution and B is
the decrement, 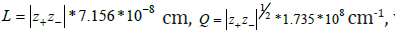 where
z+ and z+ are the charges of ions,
where
z+ and z+ are the charges of ions, 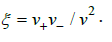
If the mixed solution is in equilibrium with the solid phase of one of the components, then in this scope the activity of the component of the solution remain constant and equal to its activity in a binary saturated solution. For example, in system NaCl-KCl-H2O (I-I valente electrolytes) we have
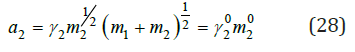
where γ20 and m20 activity coefficient and molality, respectively, in saturated solution. On the other hand, comparing the mixed solution with binary isopiestic solutions of pure components and using Eq. (8), we obtain
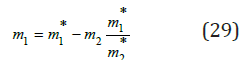
where m1* is an arbitrarily chosen concentration. Substituting Eq. (29) and (17) into Eq. (28), we can determine the concentration of the component in the mixed solution m2 by the equation
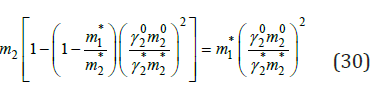
when m2* can be defined, using Eq. (21) and functions φ(x) for binary solutions of components. Further, using Eq. (24)-(27), we can define γ2*. After that, we can determine m1 according to Eq. (29). Similarly, calculation can be made when another component there is in the solid phase. Figure 2 shows the calculated and experimental data on the composition of saturated solutions in the ternary system. The component activity was calculated using Eq. (24-27); experimental data for osmotic coefficients in binary solutions were used as initial data to determine the parameters of equations [4]. These parameters were also using when the calculated concentrations were in the scope of oversaturated solutions, since the transition in this scope is not accompanied by a sudden change in the properties of the solution. Considering the ternary water-salt systems in another aspect, it is possible to evaluate the efficiency of the separation of inorganic substances during crystallization from solutions. For inorganic compounds that do not form solid solution, according Ref. [5], the co-crystallization coefficient can be defined as
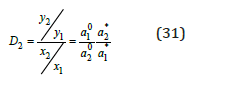
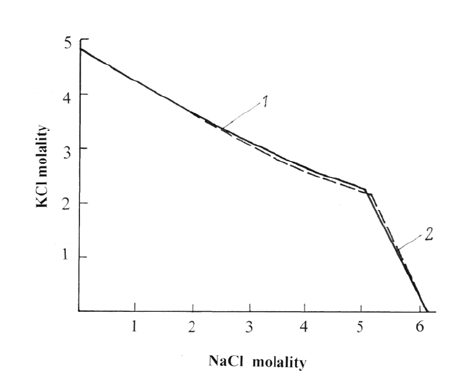
Figure 2: Solubility in the systems NaCl-KCl-H2O at 298K. 1–saturated solutions of potassium chloride. 2–saturated solutions of sodium chloride. The solid line is experimental data [4]. The dotted line is calculated data.
where yi is the molar fraction of the component in solid phase. In the limiting case when x2⤑0 also a1*⤑a10 and co-crystallization coefficient of impurity component can be expressed as
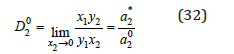
Table 1: Cocrystallization coefficients in ternary systems at 298K.
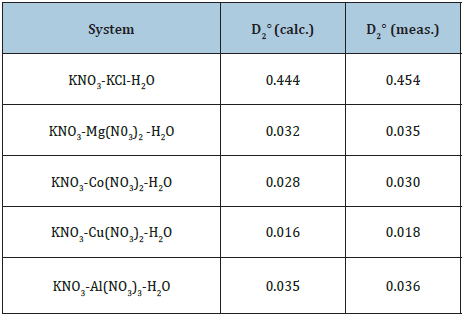
where is the activity of impurity component in its binary solution, which isopiestic to the saturated of the main component? Using Eq. (21) and functions φ(x) for binary solutions of components, we can define m2* and further γ2*, using Eq. (24). The calculated and experimental data [6] of co-crystallization coefficient for some systems, where the main component is potassium nitrate, are given in Table 1.
Conclusion
Thus, using the example of solubility and co-crystallization of components in ternary water-salt systems, the possibility of calculating the properties of ternary systems using properties of binary solution of individual components has been shown, which is of theoretical and practical interest for the technology of processing mineral raw materials.
References
- McKay HAC, Perring JK (1953) Calculations of the activity coefficients of mixed aqueous electrolytes from vapor pressures. Trans Faraday Soc 49(363): 163-165.
- Allakhverdov GR, Stepin BD (1973) Some appropriateness in thermodynamics of mixed electrolyte solutions. Zh Phys Chem (Rus) 47(2): 386-389.
- Allakhverdov GR (2019) Thermodynamics of the electrolyte solutions. AMMS 4(1): 465-474.
- Mikulin GI (1968) Current issues in the physical chemistry of electrolyte solutions. Leningrad, Russia.
- Allakhverdov GR, Zhdanovich OA (2019) Theoretical aspects of obtaining pure inorganic substances during crystallization from solutions. AMMS 3(2): 396-397.
- Allakhverdov GR, Mikhlin AL (2017) Separation factors for the crystallization of inorganic substances from aqueous solutions. Inorganic materials 53(5): 525-528.
© 2019 Allakhverdov GR. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






