- Submissions

Full Text
Aspects in Mining & Mineral Science
Influence of H2S Partial Pressure on the Corrosion Behavior of X60 Carbon Steel
Nayef M Alanazi*
Research & Development Center, Saudi Arabia
*Corresponding author: Nayef M Alanazi, Research & Development Center, Saudi Arabia
Submission: September 25, 2019;Published: December 09, 2019

ISSN 2578-0255Volume4 Issue2
Abstract
The objective of this paper is to study the influence of H2S/CO2 partial pressure ratios on the corrosion behavior of carbon steel X60. In this work, micromorphology of corrosion product was investigated by X-ray diffraction and scanning electron microscopy. The corrosion behavior of X60 steel under different conditions was analyzed. The results showed that the presence of H2S induced the formation of iron sulfide films that provide protective properties to the metal and the corrosion rate decreased with increasing the H2S/CO2 partial pressure ratios. This behaver is because of the increase in the corrosion film thickness with increasing the H2S/CO2 partial pressure ratios.
Keywords: Sour corrosion; H2S/CO2 partial pressure ratios; Carbon steel; Electrochemical techniques
Abbreviations: HIC: Hydrogen Induced Cracking; SSCC: Sulfide Stress Corrosion Cracking; CRA: Corrosion Resistant Alloys; SEM: Scanning Electron Microscope
Introduction
Sour corrosion is the most common corrosion in oil and gas industry and apart from inducing severe general and localized corrosion, the presence of H2S would cause hydrogen induced cracking (HIC) and sulfide stress corrosion cracking (SSCC) of pipelines and tubings [1-4]. Even though corrosion resistant alloys (CRA) has long been available as a material selection option that mitigates CO2 and H2S corrosion, carbon steel is in general more cost-effective for oil and gas facilities and hence, is the most widely used material option. Over the past years, number of researchers have studied the effect of hydrogen sulfide gas concentrations on the corrosion behavior of carbon steel [5-7]. Several studies have shown that the presence of H2S could either cause an acceleration or an inhibition of the corrosion of carbon steel, depending on the partial pressure of H2S [8]. Some studies also demonstrated the mechanism of H2S/CO2 corrosion of pipeline and presented certain models [9-12]. Though H2S/CO2 corrosion of pipelines has been studied widely, most of the studies focused on the characteristics of corrosion products and weight loss test after immersion. The corrosion mechanism of steel under H2S/CO2 conditions remains poor, especially in the environments with high H2S partial pressure. In addition, no clear description is available on the effects of different H2S/CO2 partial pressure ratios on the corrosion mechanism of pipeline steel. The objective of this study is to evaluate the effect of H2S/CO2 partial pressure ratios on corrosion of carbon steel in NACE solution A, and to characterize the corrosion products film.
Experimental Procedure
Corrosion tests were conducted on carbon steel 5LX60 and the sample surface was grounded to a 600-grit silicon carbide (SiC) finish. The sample was cleaned using detergent and warm water, followed ultrasonically by acetone, after which it was dried thoroughly. NACE solution A (5wt% NaCl and 0.5wt% acetic acid) was used in this work and corrosion tests were conducted at room temperature. The total H2S/CO2 partial pressure was maintained at 4.0MPa. The amounts of H2S and CO2 were adjusted to obtain the environments with different H2S/CO2 partial pressure ratios (0.01:0.99, 0.1:0.9 and 1:0) which were labeled in the figures as 1% H2S, 10% H2S and 100 H2S, respectively. The blended gas was continuously purging in the corrosion cell during the exposure time at constant rate (25ml/min). Potentiodynamic scan were utilized in-situ to measure polarization resistance and corrosion current density of produced films. The test electrode was allowed to stabilize for approximately 60min. Immediately following the stabilization period, the test electrode was polarized at a scan rate of 1.66mV/s from an initial potential of -1V(vs. OCP) to the final potential of +1V. Phase composition analysis of the corrosion film was identified by X-ray diffraction (XRD) technique utilizing a PANalytical X’Pert PRO instrument operated at 45kV and 40mA with Cu kα radiation (λ=1.5418Å). Scanning electron microscope (SEM) was employed to analysis morphology of corroded samples.
Result and Discussion
The corrosion behavior of carbon steel exposed to the H2S/CO2 environments is influenced by the structural characteristics of the corrosion products. After 96hrs of exposure, corrosion products covered the entire coupon surface. Figure 1 shows the SEM surface morphologies of the coupons exposed at different H2S/CO2 partial pressure ratios where flower-like corrosion products were observed on the surface of two coupons exposed to 1% H2S and 10% H2S but with larger size in sample exposed 10% H2S. In contrast with coupons exposed to 100% H2S, flake-like corrosion products were seen clearly on the surface. The XRD pattern in Figure 2 confirms presence of mackinawite as main corrosion product and decreases with decreasing partial pressure of H2S. The corrosion behavior of carbon steel exposed to different H2S content in NACE solution A was evaluated through the potentiodynamic polarization technique as shown in Figure 3. The polarization curves show that the corrosion behavior of formed corrosion products films is small differences but significant. The potentiodynamic polarization measurements confirm the inhibition effect of H2S on corrosion of carbon steel in the NACE solution A.
Figure 1: SEM images on the surface morphologies of the coupons exposed to a) 1% H2S b) 10% H2S and c) 100% H2S balanced with CO2 in NACE solution A at room temperature.

Figure 2: XRD pattern on the surface of the coupons exposed to different H2S/CO2 partial pressure ratios in NACE solution A at room temperature.
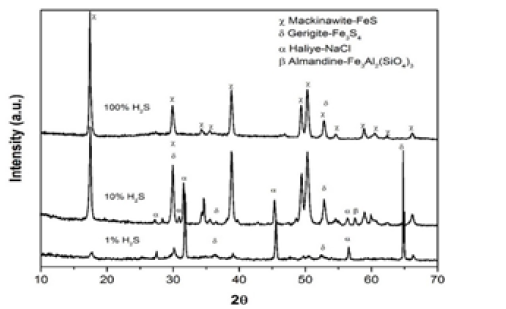
Figure 3: a) Polarization resistance and b) Corrosion current density versus immersion time for carbon steel exposed to1% H2S, 10% H2S and 100% H2S balanced with CO2 in NACE solution A at room temperature.
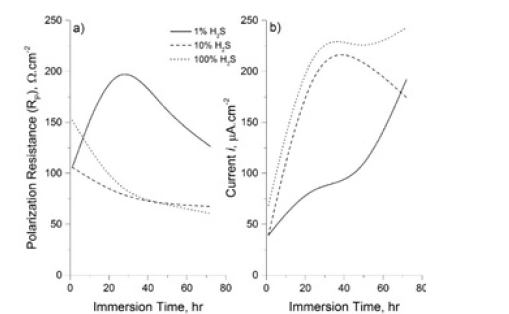
After one day of immersion, the anodic and cathodic polarization curves are exactly same trend for both H2S concentrations 10 and 100 mole%. Whilst for 1% H2S concentration, it is found that the corrosion potential of X60 was slightly higher than that obtained from other H2S/CO2 partial pressure ratios. The polarization resistance of X60 coupon exposed to 10 and 100% H2S decreases with increasing immersion time. Whilst, the polarization resistance measured on the coupon exposed to 1% H2S increased sharply until reach 24hrs of exposure then decreased suddenly with the rest of immersion time. In addition, the current densities of coupons exposed to 10% H2S, 100% H2S increased rapidly during first day of immersion by more than two times compared to the one exposed to 1% H2S. It can be remarked clearly that the corrosion rate of coupon exposed to 100% H2S was very high compared to other H2S/CO2 partial pressure ratios. XRD composition analysis for coupon exposed to 100% H2S was almost 100% mackinawite phase presence in the formed corrosion film. This can be the driving force of the observed aggressive corrosion behavior. It was reported [8] that the significant impact of precipitated mackinawite phase of iron sulfide can have in the acceleration of the localized attack.
Conclusion
In this study, the difference in corrosion behavior of carbon steel exposed to three different H2S/CO2 partial pressure ratios in NACE solution A was compared by characterizing corrosion products and analyzing electrochemical measurements. The experimental results demonstrated that the corrosion rate decreased with increasing the H2S/CO2 partial pressure ratios. However, in case of damage in corrosion films, the experimental results showed inversely where the corrosion rate increased with increasing the H2S/CO2 partial pressure ratios. The mackinawite is the most aggressive component of iron sulfide phase and it became more superiority in the corrosion products with increasing the H2S/CO2 partial pressure ratios.
References
- Nayef MA, Abdullah AA (2019) The Effect of the partial pressure of H2S and CO2 on the permeation of hydrogen in carbon steel by using pressure buildup techniques. Corrosion 75(10): 1207-1215.
- Bai P, Zheng S, Chen C (2015) Electrochemical characteristics of the early corrosion stages of API X52 steel exposed to H2S environments. Corros Sci 149-150: 295-301.
- Nayef MA, El Sherik AM, Rasheed AH, Saleh HA, Marwan RD, et al. (2015) Corrosion of pipeline steel X-60 under field-collected sludge deposit in a simulated sour environment. Corrosion 71: 305-315.
- Zhou C, Zheng S, Chen C, Lu G (2013) The effect of the partial pressure of H2S on the permeation of hydrogen in low carbon pipeline steel. Corros Sci 67: 184-192.
- Saenz De M, Turnbull A (1989) The effect of H2S concertation and pH on the cracking resistance of AISI 410 stainless steel in 5% brine. Corros Sci 29(1): 69-88.
- Veloz MA, González I (2002) Electrochemical study of carbon steel corrosion in buffered acetic acid solutions with chlorides and H2 Electrochim Acta 48(2): 135-144.
- Aezola S, Genesca J (2005) The effect of H2S concentration on the corrosion behavior of API 5L X-70 steel. J Solid State Electrochem 9(4): 197-200.
- Tang J, Shao Y, Guo J, Zhang T, Meng G, et al. (2010) The effect of H2S concentration on the corrosion behavior of carbon steel at 90 ⁰C. Corros Sci 52(6): 2050-2058.
- Hends G, Turnbull A (2010) Novel multi-electrode test method for evaluating inhibitor of under-deposit corrosion-part 2: Sour conditions. Corrosion 66(5): 056002-056002-6.
- Yin ZF, Zhao WZ (2008) Corrosion behavior of SM 80SS tube steel in stimulant solution containing H2S and CO2. Electrochimi Acta 53(10): 3690-3700.
- Li WF, Zhou YJ, Xue Y (2012) Corrosion behavior of 110S tube steel in environments of high H2S and CO2 J Iron Steel Res Int 19(12): 59-65.
- Ren CQ, Liu DX, Bai ZQ, Li TH (2005) Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide. Mater Chem Phys 93(2-3): 305-309.
© 2019 Nayef M Alanazi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






