- Submissions

Full Text
Aspects in Mining & Mineral Science
Metals and Metallurgy
Fathi Habashi*
Department of Mining, Metallurgical and Materials Engineering, Canada
*Corresponding author: Fathi Habashi, Department of Mining, Metallurgical and Materials Engineering, Laval University, Canada
Submission: March 20, 2019;Published: May 29, 2019

ISSN 2578-0255Volume3 Issue1
The Commercial Classification of Metals
Elements can be arranged conveniently in the Periodic Table and described as metals, non-metals, and metalloids. Metals are further classified as typical and less typical, transition and inner transition (Figure 1). While this classification is useful for the chemists and physicists it does not show the economic value of the metals. For the metallurgist however, a more useful form is the commercial classification of metals: ferrous and nonferrous (Figure 2). This classification is well justified because the annual production of iron and steel in one year exceeds the production of all other metals combined in about ten years. Ferrous metals include wrought iron, cast iron, and steel. Nonferrous metals, on the other hand, is classified into primary, secondary, light, precious, etc., as shown in (Table 1).
Table 1:Commercial classification of nonferrous metals and metalloids.
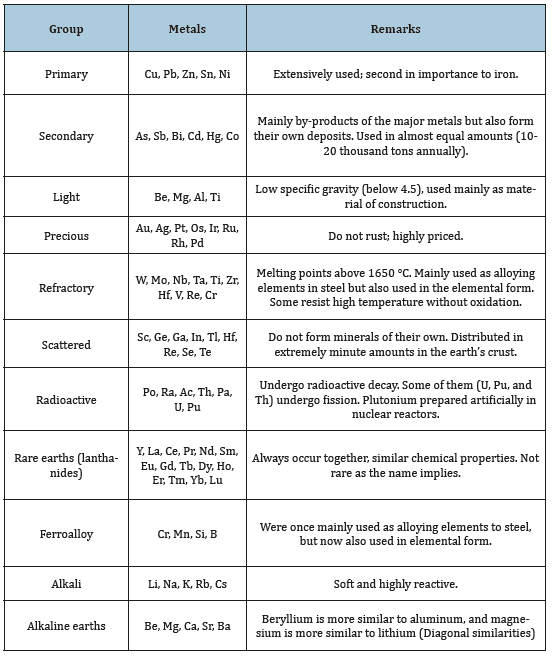
Figure 1:Periodic table showing metals, nonmetals, and metalloids and the different types of metals.
Figure 2:Ferrous and nonferrous metals.

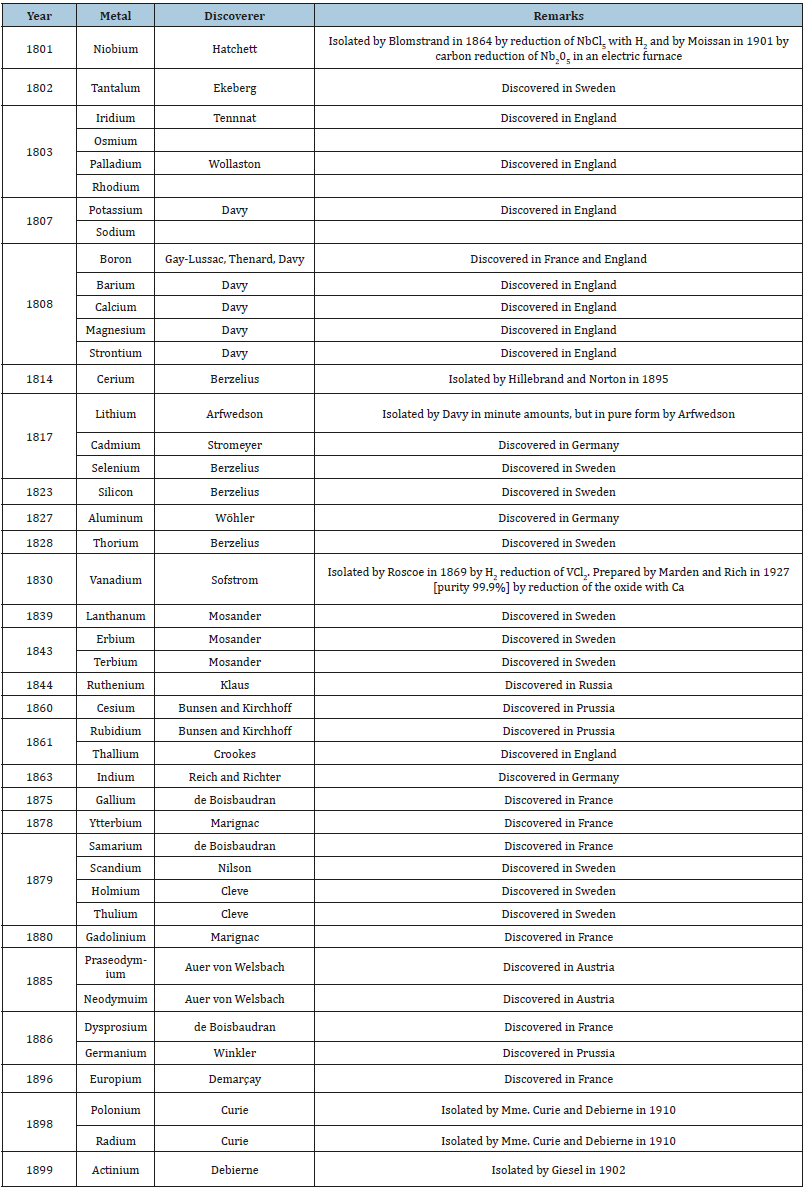
Discovery of Metals
Seven metals were used by the ancient people, then in the Middle Ages the alchemists knew aqua regia for dissolving gold and in the 13th and 14th centuries three metalloids: arsenic, antimony, and bismuth were discovered. The East supplied two metals: zinc and boron in form of borate. The bulk of metals became known in the 17th and 18th centuries.
Smelting
In the 17th century, attempts were made to understand the nature of fire and the smelting process. It was once believed that when coal was burnt, phlogiston which in Greek means flame [1], was released and a calx, that is, ash remained. If an ore or an oxide was heated with coal it takes up the escaping phlogiston in the fire to form the metal:
Ore (oxide)+Phlogiston (from coal) →Metal
It was the French chemist Antoine Laurent Lavoisier (1743- 1794) who in 1772 finally directed the fatal blow to the theory, when a few years earlier oxygen was discovered, and he interpreted the phenomenon of combustion as an oxidation process.
Tools of Discovery
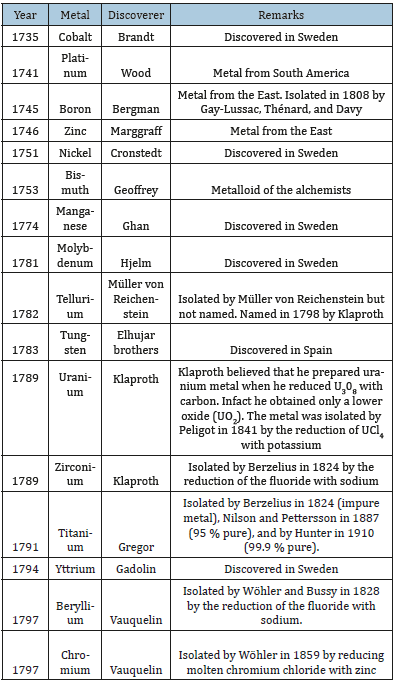
The platinum metals became known in Europe from Ecuador in South America and this was followed by many other metals (Table 2). The blowpipe (Figure 3) played an important role in the discovery. In the 19th century the metals discovered was a result of the discovery of electric current and the development in analytical chemistry and chemical theory.
Table 2:Metals discovered in the eighteenth century.
It was Robert Bunsen (1811-1899) at the University of Heidelberg, who paved the way for the great discoveries in the 19th century (Table 3) with his invention of the burner in 1852 that carries his name and is found today in most chemical laboratories (Figure 4). This burner permitted higher temperature to be achieved in the laboratory when conducting a test. Before this burner [2] the flame of a candle or from alcohol was used. The Bunsen burner flame permitted performing the flame tests (Figure 5) and later spectroscopic analysis (Figures 6 & 7).
Figure 3:The blowpipe.
Figure 4:Bunsen burner.
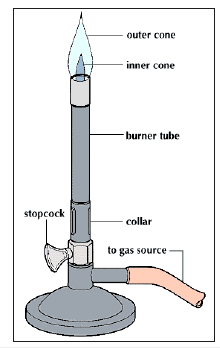
Figure 5:Examples of coloured flames in flame tests.
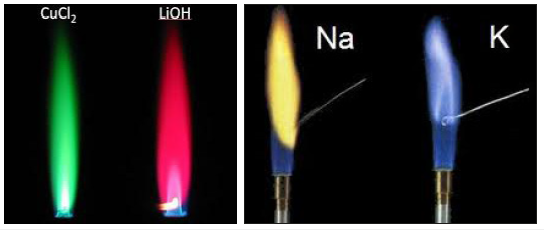
Figure 6:A first spectroscope.
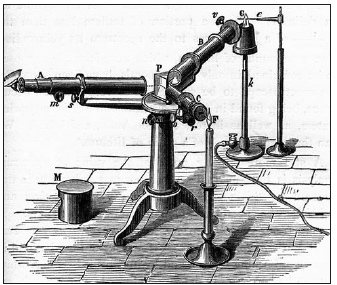
Figure 7:Examples of emission spectra: calcium, strontium, and barium.
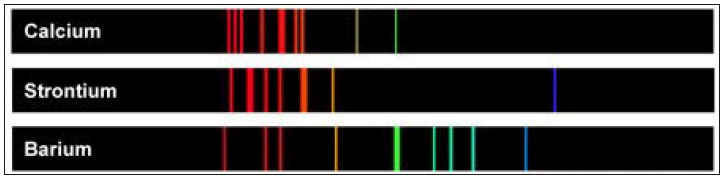
Table 3:Metals discovered and isolated in the nineteenth century.
The Industrial Production of Aluminum
A great technological advance was the invention of the dynamo in the 1870’s that made available electricity in bulk which encouraged the expansion of electrolytic copper refining to supply the pure copper needed for the electrical industry. Another important application of electricity was in the electrolytic production of aluminum. Once aluminum was available inexpensively, it was used for reducing other oxides to metals. Thus, chromium and manganese were prepared a few years later by this technique.
X-Rays and Radioactivity
In 1895 x-rays were discovered by Wilhelm Conrad Roentgen (1845-1923) followed by the discovery of radioactivity in 1898 by Antoine Henri Becquerel (1852-1908) which was responsible for the discovery of polonium and radium [3] shortly afterwards by Marie Curie (1867-1934). A year later, André Debièrne (1874- 1949) a co-worker with Curie discovered and isolated actinium.
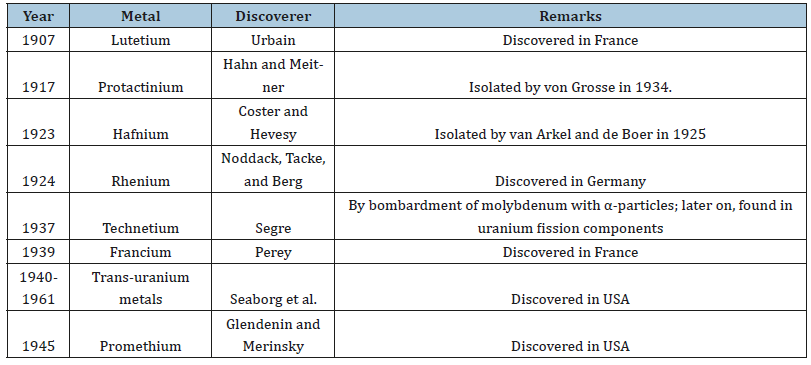
Metals of the Twentieth Century
Elements discovered in the 20th century are those which are very rare or do not occur in nature (Table 4). Elements beyond plutonium were discovered at the Lawrence Radiation Laboratory, University of California from 1944 to 1961 by Seaborg, McMillan, and Ghiorso:
A. 1944, americium and curium
B. 1949, berkelium
C. 1950, californium
D. 1952, einsteinium
E. 1956, nobelium
F. 1961, lawrentium
Table 4:Twenty century metals.
Development of Radiochemistry
Radiochemists played an important role in separating the individual radioelements, their identification, and their arrangement in series. New methods of measurements such as the Geiger counter and the gamma scintillation counter replaced the gold leaf electroscope. Radiations from radioactive substances were identified as alpha, beta, and gamma. As a result, two very rare radioactive metals: protactinium and francium were discovered. Protactinium was discovered in 1917-18 by Otto Hahn (1879- 1968) and Lise Meitner (1878-1968) in Germany the new element was part of the decay chain of uranium-235.
Francium was discovered in 1939 at Curie Institute in Paris, France by Marguerite Perey (1909-1975) from which the element takes its name. It was discovered during the purification of a sample of actinium 227. It is extremely rare, with trace amounts found in uranium and thorium ores, where the isotope francium-223 continually forms and decays.
The Discovery of Lutetium
Lutetium, element 71, was discovered spectroscopically in 1907 by the French chemist Georges Urbain (1872-1938) (Figure 3) who named it after Lutia the Roman name of the place where Paris was founded. It was identified by Bohr as a rare earth and not as a member of Group IV.
Quantum Theory
The quantum theory by Max Planck (1858-1947) is based on the principle that energy like matter is also composed of minute quantities called quanta, i.e., energy is not continuous but occurs in small parcels. This allowed the understanding of the movement of electrons [4] in the atom by Niels Bohr (1885-1962), the hypothesis of the formation of electron shells, and the explanation of emission spectra.
Electronic Structure of Rare Earths
In 1922 Bohr elucidated the electronic structure of the rare earths. The 14 rare earth elements were identified as “inner transition metals” and were assigned a special place in the Periodic Table that became known as “lanthanides”. In this group the two outermost electronic shells are filled with the same number of electrons, and it is in the third shell that the number of electrons is increased gradually. This explained the close similarity of the members of this group in their chemical behavior. Bohr concluded that element 72 which occurs after lutetium must be tetra-valent rather than trivalent and must belong to the zirconium family and not the rare earths. He advised his co-workers in his laboratory to search for this element in zirconium ores.
X-Ray Analysis
X-ray spectrum analysis by Henry Moseley (1887-1915) in 1914 led to the discovery of two metals: hafnium and rhenium.
Discovery of The Neutron
James Chadwick (1891-1974) in Cambridge in 1932 explained the experiments of Frédéric Joliot (1900-1958) and Irène Curie (1897-1957) in Paris by supposing that alpha particles were knocking neutral particles out of the nuclei of the beryllium atom and that these neutral particles were in turn knocking protons out of the paraffin. In this way the neutron was discovered.
Neutron Capture
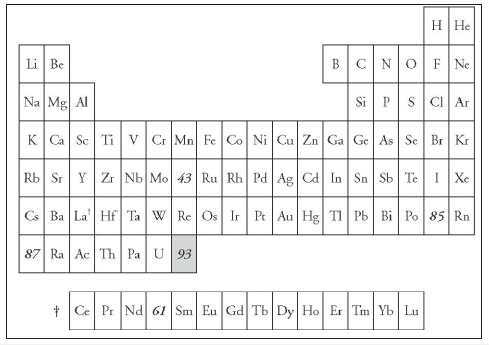
In 1934, Enrico Fermi (1901-1954) in Rome discovered that neutrons may be captured by atoms and that the frequency of capture increases when they are slowed down by passing them through a hydrogen-rich material such as paraffin or water. He was thus able to produce atoms of higher atomic weights than those bombarded. For example, on bombarding cobalt with neutrons he was able to produce nickel. When, however, he and his coworkers bombarded uranium with neutrons, they obtained more than one radioactive product. Following the same line of thought as in their previous experiments they suggested that one of these products was formed by neutron capture, i.e., that it was a trans-uranium element or element number 93. Fermi put the new element under rhenium in the Periodic Table and called it eka-rhenium (Figure 8).
Fermis’ paper naturally attracted the attention of Ida Noddack the discoverer of rhenium because it dealt with another element in the manganese group. Soon afterward, she published a paper which showed that Fermi’s experimental evidence was incomplete. Her argument was as follows: when atoms are bombarded by protons or alpha particles, the nuclear reactions that take place involve the emission of an electron, a proton, or a helium nucleus and the mass of the bombarded atom suffers little change. When, however, neutrons are used, new types of nuclear reaction should take place that are completely different from those previously known It would be reasonable to propose that they break down into numerous large fractions. Here she conceived the idea of nuclear fission.
Figure 8:Eka-rhenium according to Fermi, 1934.
Discovery of Uranium Fission
Fermi’s experiments were repeated by Otto Hahn (1879-1968) and his coworkers in Berlin. They confirmed Fermi’s conclusions and published a series of papers on extensive radiochemical separations of the so-called trans-uranium elements. The results, however, became so contradictory that after five years of intensive research and extensive publication the concept of trans-uranium elements had to be abandoned. Hahn then announced in January 1939 the definite formation of barium during the bombardment of uranium and started speculating about the mechanism of its formation. Hahn could not accept the new idea that the uranium atom was split into two fragments. It was Lise Meitner in Sweden who finally explained the results of the work as fission, a few months after she was forced to leave Germany in 1939.
Cyclotron
The cyclotron was invented by Ernest O. Lawrence (1901-1958) of the University of California, Berkeley, where it was first operated in 1932. By its means technetium and the trans-uranium metals were discovered. In 1937 the Italian physicists Emilio Segrè (1905- 1989) and his co-worker C. Perrier announced the detection of the element with atomic number 43 in trace amounts in a molybdenum target which has been bombarded in the cyclotron for several months with a strong deuteron beam. They called this new element technetium deriving the name from the Greek word for “artificial”.
Transuranium
After elucidating the electronic structure of the trans-uranium which resembled that of the lanthanides, Seaborg (1912-1999) proposed a second series of inner transition metals similar to the lanthanides that became known as “actinides”. He changed the Periodic Table of 1945. Thus, uranium was removed from Group VI to become a member of this new group.
Promethium
The existence of a rare earth element between neodymium and samarium was predicted by Brunauer. This was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was no element with atomic number 61. This element was discovered by Glendenin, Marinsky, and Coryell in 1945 at Oak Ridge National Laboratory in uranium fission products and named “promethium”. Promethium does not occur in nature.
Metallurgy in the Past Decades
Word War II ended by the use of atomic bomb. The bomb was the result of Manhattan Project in USA in 1940s which played an important role in developing extractive metallurgy for the production of uranium. It was responsible for advancing the technology of metallothermic reactions, introducing new leaching processes, new precipitation methods, new reagents, ion exchange technology, and solvent extraction.
For the peaceful uses of atomic energy, uranium and transuranium elements were thoroughly studied, and the chemistry of other metals such as beryllium, boron, cadmium, zirconium, hafnium, rare earths, etc., became widely known. For the sake of conveniently outlining these developments, extractive metallurgy is divided into three sectors: pyro-, hydro-, and electrometallurgy. However, it is not possible to separate the three sectors since in general all three may be involved in the recovery of a particular metal.
References
- Habashi F (2003) Metals from ores: An introduction to extractive metallurgy. Published by Métallurgie Extractive Québec, Québec City, Canada.
- Habashi F (2010) Metals: typical and less typical, transition and inner transition. Foundations of Chemistry 12(1): 31-39.
- Habashi F (2015) The story of metals, two volumes. Published by Métallurgie Extractive Québec, Québec City, Canada.
- Habashi F (1997) Handbook of extractive metallurgy, Wiley-VCH, Weinheim, Germany, Vol. 4.
© 2019 Fathi Habashi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






