- Submissions

Full Text
Associative Journal of Health Sciences
A Technology-Driven, Interprofessional Pandemic Response Strategy for a Comprehensive Academic Health Center
Chad Douglas, Eric Edwards and Susan E Conway*
Pharm. D, University of Oklahoma Health Sciences, Oklahoma, USA
*Corresponding author:Susan E Conway, Pharm. D, University of Oklahoma Health Sciences, 1110 N Stonewall, Room 135, Oklahoma City, OK 73117, USA
Submission: April 23, 2024;Published: April 29, 2024

ISSN:2690-9707 Volume3 Issue2
Abstract
Objective: Describe a technology-driven, multifaceted COVID-19 pandemic response strategy for a
comprehensive academic health center.
Population: The University of Oklahoma (OU) Health Sciences Center and the OU Health hospital system,
separate legal entities together referred to as the comprehensive academic health center, has an average
population of 12,000 employees and 2,600 students.
Methods: Key components of the COVID-19 response strategy included campus policies, screening and
reporting tool, testing program, and immunization clinics.
Result: A total of 46,066 forms were submitted to the online COVID-19 screening and reporting tool
and 13,618 were referred for testing. Thirty-seven vaccination clinics delivered 21,615 COVID-19 vaccine
doses to provide the primary series to employees and students.
Conclusion: An interprofessional, collaborative effort successfully mitigated risk throughout the
COVID-19 pandemic using mass vaccination efforts and a coordinated reporting, screening, and testing
program at this comprehensive academic health center.
Keywords:Severe acute respiratory syndrome coronavirus-2; SARS-CoV-2; COVID-19; Risk mitigation; exposure management; COVID-19 vaccine; Comprehensive academic health center
Introduction
The coronavirus was first identified in Wuhan, China, and rapidly spread throughout the world, primarily due to social distance proximity and uncontrolled travel [1]. After widespread infection of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in more than 114 countries, the World Health Organization (WHO) declared the outbreak a pandemic on March 11, 2020 [2]. An overview of the COVID-19 pandemic from a clinical and transmissibility perspective supports the critical need for a robust risk mitigation response to ensure the health and safety of a large campus community.
SARS-CoV-2 infections have a similar presentation to severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS), though SARS-CoV-2 has a higher rate of transmission. Infected patients often present with influenza-like symptoms including fever, cough, and muscle aches. Severe SARS-CoV-2 infections can progress to pneumonia, acute respiratory distress syndrome, and multi-organ failure [3]. Once the identification of the virus was confirmed, viral genomic sequencing was performed; this facilitated the development of diagnostic molecular testing methods to confirm SARS-Co-V-2 infections.
Transmission of SARS-Co-V-2 was determined to be human-to-human through respiratory droplets, though airborne transmission could occur in certain circumstances [4]. Spread of the virus through contact with contaminated surfaces was determined to be much less significant than other methods of transmission. Recommendations to control the transmissibility of the virus were to increase distance between individuals (6-foot social distancing) and to encourage sanitization of hands, disinfection of fomites, and wearing masks to control the spread of respiratory droplets.
As the pandemic progressed, several treatments received Emergency Use Authorization (EUA) and some were eventually granted full approval from the Food and Drug Administration (FDA). In December 2020, nine months into the pandemic, the first two vaccines received EUA approval for prevention of COVID-19. Throughout the pandemic, the Centers for Disease Control and Prevention (CDC) led efforts to mitigate COVID-19 infection through timely issuance and updates to clinical guidelines, as well as recommendations including key components such as vaccination, masking, social distancing, and testing. The CDC guidance shaped the formation of state and local policies including for individual health systems and universities.
The purpose of this article is to describe a technology-driven, multifaceted, and interprofessional COVID-19 pandemic response implemented at a comprehensive academic health center using the collaborative efforts of administrators, faculty, staff, and students to operationalize guidance from the CDC in order to mitigate the spread of COVID-19. The pandemic response strategy described focuses on four key components: committee governance, symptom and exposure reporting and screening tool, onsite testing, and vaccination. This strategy highlights essential steps and unique features that enabled our comprehensive academic health center to develop a comprehensive, efficient, and effective pandemic response for a large campus population.
Population
Our comprehensive academic health center is comprised of The University of Oklahoma (OU) Health Sciences Center and its health system partner OU Health. The OU Health Sciences Center is comprised of seven professional colleges: Allied Health, Dentistry, Medicine, Nursing, Pharmacy, Public Health, and the Graduate College and has a student population of approximately 2,600. OU Health provides comprehensive healthcare services through three hospitals; 85 clinics; and diabetes and cancer centers. The combined employee population at OU Health Sciences Center and OU Health is approximately 12,000.
Committee governance
Given the intersecting operations of the two entities that comprise our comprehensive academic health center, the pandemic response was a collaborative effort designed to protect our employees and students, as well as the patients that this comprehensive academic health center serves. Committee work combined the efforts of the OU Health Sciences Center’s Specific Pathogens Preparedness Operations Team and Emergency Operations Committee as well as OU Health’s Specific Pathogens Operations Response Team and Incident Command Committee. The collaborative committees included representation from both entities from senior administration, operations, human resources, legal counsel, public health faculty, infectious diseases faculty, employee health, student health, clinical practices and information technology (IT). These committees were tasked with the development, implementation, evaluation, and modification of the campus-wide COVID-19 response policies and protocols that would apply to all health care providers, faculty, staff, and students. Each component of the response plan targeted important strategies to mitigate COVID-19 risk for the collective population. The COVID-19 response plan was designed to address remote working and learning, social distancing, masking, screening, reporting, testing, quarantine, isolation, exposure management, and vaccine distribution and administration. The committees’ independent and coordinated work focused on implementing the CDC’s COVID-19 guidelines as well as adapting these recommendations to our state and local specific phases of the pandemic, with the goal of decreasing the severity of disease burden.
Screening and reporting tool
Within a matter of weeks of the onset of the pandemic, Employee and Student Health implemented an electronic screening and reporting tool that was used to identify and manage those individuals with high-risk travel, exposures, symptoms, and confirmed positive COVID-19. The creation of the tool was a collaborative effort between Employee and Student Health and the IT Department, with guidance from the Emergency Operations Committee and Incident Command Committee. This tool consisted of a data collection form and risk assessment algorithm based on CDC guidelines to determine if an employee or student could safely remain on or return to campus. This tool also helped determine if quarantine, isolation, symptom monitoring, and/or COVID-19 testing would be required and facilitated with the monitoring, follow up, and clearance of individuals to safely return to campus following an exposure or positive test. The primary goal of this screening and reporting tool was to mitigate spread of COVID-19 to and among students, employees, and patients.
Employees and students who experienced within the previous 14 days one or more CDC-defined symptoms that could be associated with a SARS-Co-V-2 infection were required to complete the online electronic screening form. Completion of the form was also required for employees and students without symptoms who had a direct exposure to a confirmed COVID-19 case within the previous 14 days, as well as for those employees and students who had recently tested positive for COVID-19 at an off-campus testing site. Additional screening questions included the date of symptom onset, recent known COVID-19 exposures, and specific personal protective equipment worn during an exposure. The form also included questions regarding the potential contact a symptomatic or known positive COVID-19 employee or student had with coworkers and other students to assist with exposure management for our campus. Travel screening was a component of the tool in the early stages of the pandemic and focused on travel to or from areas with high or escalating COVID-19 cases as well as cruise travel. Specific travel screening was eventually discontinued when cases in our state and local community rose to match those elsewhere.
Beginning in early 2021, questions were added to incorporate vaccination status, including the number, type, and dates of COVID-19 vaccines received into the risk assessment algorithm. This risk assessment was then utilized to provide CDC congruent guidance to employees and students with exposures regarding quarantine, testing, follow-up, and clearance to return to campus.
Once the employee or student completed and submitted the electronic form, the screening tool algorithm performed a risk assessment calculation and sent a secure email response to the individual specifying whether they were cleared or not cleared to return to campus. The forms submitted by employees and students who were denied clearance to return to campus were subsequently evaluated by the Employee and Student Health clinicians. These forms were transformed into cases and stored within a secure platform to document communications and facilitate follow-up with the employee or student. Employee and Student Health would then provide these individuals with additional instructions regarding quarantine, isolation, symptom monitoring, and testing, per campus policy. The campus COVID-19 policy and screening tool algorithm required routine updates based on the evolving CDC guidelines but remained conservative throughout the pandemic. This conservative approach was deemed necessary by the governance committees, given the fact that most employees and students either had direct patient contact or were no more than one person removed from direct patient care.
Employee and Student Health protocols derived from campus COVID-19 policy were incorporated into the risk assessment algorithm and utilized by the assessing clinicians. These specified management strategies and categorized individuals by symptoms, exposures, test results, and vaccination status. There were defined criteria for each of these subcategories that had to be met for an individual to be cleared to return to campus. Examples of criteria to return to campus after an exposure included date of exposure, length of exposure, personal protective equipment worn during the exposure, ongoing household or one-time community or workplace exposure, vaccination status, and subsequent COVID-19 testing results. A status category was assigned to each individual as shown in Figure 1. Return to campus criteria for those who tested positive for COVID-19 followed CDC guidelines and included time since symptom onset or positive test, severity of illness, afebrile period while abstaining from the use of anti-pyrectics, and improvement in symptoms.
Figure 1:Screening and Reporting Tool Sample Dashboard- Home Quarantine (HQ) and Self Isolation (SI) Status. SOC- Symptoms of Concern.
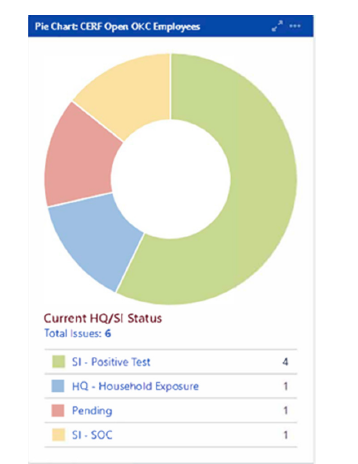
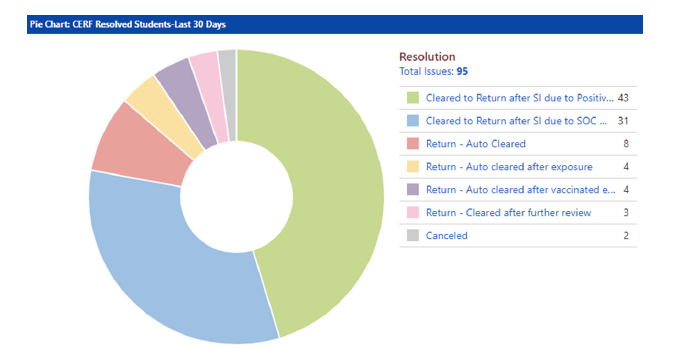
Screening and reporting tool data reports were routinely reviewed by the governance committees, and campus trends were assessed using these reports and a real-time data dashboard feature built into the tool. A sample from the dashboard is shown in Figure 2. This data provided crucial information to the governance committees to inform policy updates, especially those relating to remote work and learning and the return to campus plan. This information also provided real-time statistics to monitor workplace shortages and keep the governance committees informed of the number of COVID-19 cases, quarantines, and isolations in the campus and hospital population.
Figure 2:Screening and reporting tool sample dashboard- case resolution status. SI- Self Isolation, SOC- Symptoms of Concern.
Onsite COVID-19 testing
The COVID-19 campus testing program began in March 2020 as a strategy to provide easily accessible, timely, and reliable testing for our employees and students with symptoms and those with potential COVID-19 exposures. The screening and reporting tool identified individuals considered to be at risk for COVID-19, and these employees and students were required to be tested before returning to campus responsibilities. The testing program was designed by the medical directors of OU Health Employee Health and OUHSC Employee and Student Health, was scheduled by OUHSC IT-created systems, and was operated by OU Health. OU Health laboratory processed test results, and OUHSC Employee and Student Health managed communication with individuals regarding test results, isolation, follow-up, and clearance. Test results were entered into the respective employee’s or student’s screening form case in order to utilize an automated follow-up and clearance system.
The testing site was located on campus and offered 40 testing appointments per day initially; as the pandemic expanded, the capacity for testing was increased and maximized at 100 test appointments per day. As a measure to decrease risk of exposure between the potential case and test administrator as well as to socially distance potential cases, drive-thru testing was the primary process employed. The primary testing site was available to students and employees from 8 am to 5 pm Sundays through Fridays. Additionally, testing was available on Saturdays for employees who were seeking clearance to return to work on Mondays. Due to the high volume of tests administered, staff rotated in shifts to confirm, document, and communicate test results and as a result were able to provide results to individuals within 24 hours. Consistent with CDC recommendations, Polymerase Chain Reaction (PCR) testing was performed on specimens collected with nasopharyngeal swabs. Testing was provided at no cost to employees and students.
From March 1, 2020, to July 1, 2022, a total of 46,066 online screening forms were submitted through the screening and reporting tool. Of those, 13,618 tests were indicated based on the algorithmic assessment; thus approximately 30% of the forms submitted required testing. The implementation of high-quality, timely testing was crucial to the success of the screening and reporting tool, and its utilization was an additional measure to maintain the effectiveness of exposure management.
Vaccination program
The purpose of the vaccination program was to promote disease prevention and improve accessibility to the COVID-19 vaccines for the comprehensive academic health center community. Mass vaccination efforts played a key role in the campus response plan. Key factors for mass vaccination efforts included vaccine availability, efficacy, and safety as well as patient preferences [5]. Given the campus community largely consisted of health care workers and health sciences students, it was critically important to have a robust vaccination initiative team that was ready to quickly distribute vaccine allocations as they were received, beginning in December 2020 following EUA from the FDA and recommendations from the CDC.
The planning for the COVID-19 immunization roll-out was led by the OUHSC College of Pharmacy, with critical leadership from a vaccine committee with representation from both OU Health Sciences Center and OU Health. The vaccine committee included department representatives ranging from hospital and campus administrators; the Pharmacy, Medicine, Nursing, and Public Health colleges; and IT, legal counsel, risk management, and public relations for both entities. The vaccine committee advised on vaccine recipient priority lists, storage, clinic locations, record keeping, vaccination clinic staffing and logistics, safety issues, scheduling, and communications. The College of Pharmacy was experienced in mass vaccine clinics through its experience in leading the campus-wide influenza vaccine efforts for the preceding twelve fall seasons, including in 2020’s influenza vaccine clinic to which they added COVID mitigation strategies. COVID-19 vaccine clinics began with nearly a daily cadence in mid-December 2020, immediately following receipt of initial shipments of Pfizer BioNTech and Moderna COVID-19 vaccines.
Primary series: The Oklahoma State Department of Health (OSDH) provided the framework for the COVID-19 vaccine rollout with regard to general vaccine priorities. Phase 1 included subphases that encompassed long-term care residents and workers, select hospital staff at high-risk for exposure, and public health staff on the front-line of the pandemic response. The College of Pharmacy led the COVID-19 vaccine Point of Dispensing (PODs) or clinics, with guidance from the collaborative vaccine committee. Vaccine inventory, storage, and preparation was managed by the College of Pharmacy’s Nuclear Pharmacy department. The vaccine committee managed the priority lists and communications to the eligible vaccine recipients. The POD staffing model was primarily supported by College of Pharmacy faculty, staff, residents, and students with contributions of many campus collaborators including the Employee and Student Health Medical Director; Chief COVID Officer; IT; Colleges of Nursing, Medicine, and Allied Health students and faculty; health-system pharmacists; healthsystem nurses; and marketing/communications staff. Vaccine documentation was data entered into the state vaccine registry initially by nursing staff and students; later OUHSC IT worked with OSDH to develop processes for automating the upload between the local documentation system to the State registry. Approximately 14,300 COVID-19 vaccines were administered in Phase 1 within 22 PODs scheduled from December 16, 2020, through January 13, 2021.
In mid-January 2021, vaccine PODs were offered to individuals identified by OSDH in Phase II, which included first responders, all healthcare workers, adults 65 and older, adults of any age with comorbidities, schoolteachers and their support staff, and other public health support staff. The PODs continued to be offered multiple times per week to vaccinate all members of the comprehensive academic health center community who had not been identified or included in Phase I. From mid-January 2021 to early-April 2021, an additional 15 PODs provided an additional 7,315 COVID vaccines to eligible employees and students.
Phases III and IV opened vaccine availability to the remaining adult populations. Vaccination efforts transitioned to community outreach clinics focused on our health-systems patients and on local under-represented communities.
Booster doses: Once COVID-19 booster doses were approved, fall influenza clinics were expanded to offer boosters as well, making getting both vaccines more convenient to vaccine recipients, presumably increasing the number of those receiving the booster. The College of Pharmacy led these clinics, offering 10 PODs throughout October 2021 and 2022, with 4,305 boosters administered in 2021 and 1,697 boosters administered in 2022. The campus pharmacies also offered COVID vaccine to those employees and students who did not come to the scheduled PODs.
Experiential training: Vaccine POD staffing models centered around providing experiential learning opportunities for our health sciences students, namely pharmacy and nursing. The College of Pharmacy faculty developed single-day Introductory Pharmacy Practice Experiences (IPPE) for first- and second-year students, 30- hour IPPE rotations for third year students, and 160-hour Advance Pharmacy Practice Experience (APPE) rotations or single-day volunteer shifts for fourth-year students. A total of 129 pharmacy students contributed over 2,000 hours to the campus COVID-19 vaccine PODs. The nursing college used a single-day experiential assignment model and recorded 100 nursing students contributing 750 hours.
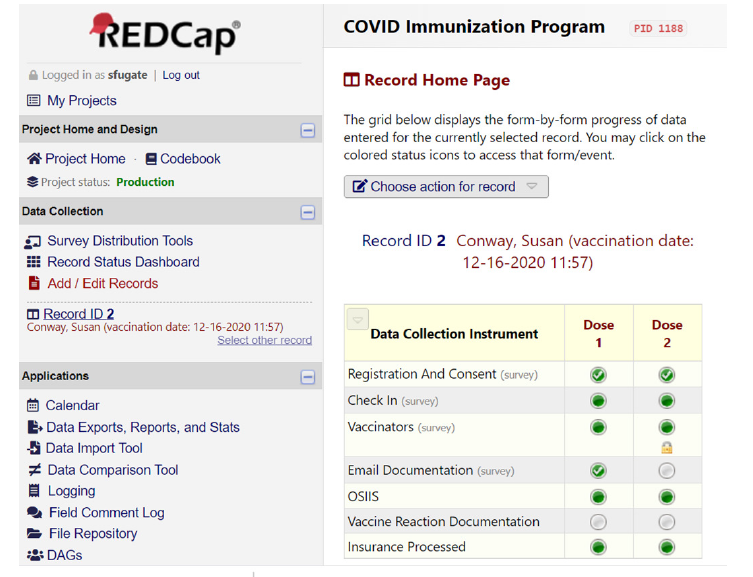
Technology systems: Campus IT used the Microsoft Bookings software tool to develop a schedule system for vaccination appointments to manage vaccine priority groups and traffic flow for the PODs. For vaccine documentation, the pharmacy faculty collaborated with the IT department to develop a tool within REDCap, a web-based application used to capture clinical data on a secure platform. REDCap served as the electronic tool that managed registration, consent, vaccine documentation, adverse drug events (ADE), and state registry documentation (Figure 3). REDCap registration collected information prior to the vaccination appointment, which included demographics, pre-vaccination screening questions and insurance information. The screening questions were modified as COVID-19 vaccine information and recommendations evolved to inform whether patients met eligibility criteria for the phase of vaccine roll-out and to ensure absence of contraindications. The vaccinator was able to review information gathered on the registration portion of the form to properly screen each individual being vaccinated. After the vaccinator documented the vaccine administration, REDCap automatically sent an email documentation of vaccine to the patient. Patient information stored within REDCap was then uploaded to the state registry, Oklahoma State Immunization Information System (OSIIS). During Phase I-IV rollouts, patients were monitored onsite by health professionals and trained volunteers for 15 minutes, consistent with CDC recommendations. Vaccine recipients who developed an Adverse Drug Event (ADE) during this period were managed in a nearby observation room by medicine, pharmacy, and/or nursing faculty. Documentation of the vaccine ADE was added to REDCap and was reported to the FDA’s Vaccine Adverse Event Reporting System (VAEARS). Vaccine documentation was shared with the Employee and Student Health department, as authorized by the registration consent process. The REDCap tool provided easy accessibility to look up vaccine recipient information later when needs arose, such as for providing documentation of ADEs or providing replacement vaccine.
Figure 3:REDCap homepage.
Conclusion
The COVID-19 response plan was successfully implemented on a comprehensive academic health center campus. The collaborative university and health-system efforts led to the development of a screening and reporting tool, campus testing program, and immunization program. The immunization program administered 27,617 doses. The screening and reporting tool was used to provide guidance to those with symptoms and those who experienced high risk exposures, and to identify individuals who required quarantine, isolation, and/or testing. The tool also facilitated exposure management efforts. This manuscript is meant to serve as a tool for other comprehensive academic health centers to educate, guide, and provide a foundation for strategic planning in the event of future public health pandemics.
Acknowledgement
The authors would like to acknowledge all our colleagues and students who contributed to a successful campuswide COVID-19 response with a special acknowledgement for Dale Bratzler, D.O., M.P.H.; Vince Dennis, Pharm.D.; Bruce Dobey, PA-C, M.H.S.; G.T. Dolan, Pharm.D.; Doug Drevets, M.D.; Michael Hines, MBA; David Horton; Eric Johnson, CPA, M.BA, M.S.-MIS; Michael Kennedy; Kristina Kline, M.D.; Chris Kobza; Teresa Lewis, Pharm.D.; Lynn Mitchell, M.D., M.P.H.; Jill Raines, J.D., LL.M.; Kevin Rinaldi, MBA; Linda Salinas, M.D.; Kate Stanton, M.H.R.; Katherine O’Neal, Pharm.D., MBA; Todd Tucker; Donna Tyungu, M.D.; Aaron Wendelboe, Ph.D.; Megan Westbrook, MBA; and Thomas Wilson.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2019) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8): 727-733.
- Cucinotta D, Vanelli M (2020) WHO declares COVID-19 a pandemic. Acta Biomed 91(1): 157-160.
- Sharma A, Tiwari S, Deb MK, Marty JL (2020) Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents 56(2): 106054.
- Lotfi M, Hamblin MR, Rezaei N (2020) COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clinica Chimica Acta 508: 254-266.
- Edwards KM, Hackell JM (2016) Countering vaccine hesitancy. Committee on infectious diseases, the committee on practice and ambulatory medicine. Pediatrics 138(3): e20162146.
© 2024 Susan E Conway. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






