- Submissions

Full Text
Associative Journal of Health Sciences
Myocarditis as a First Manifestation of Rheumatoid Arthritis
Zahidi HA*, Charfo M, Atlas I, Haboub M, Arous S, EM Benouna, Drighil A, Azzouzi L and Habbal R
Department of Cardiology, IBN ROCHD Casablanca University Hospital, Morocco
*Corresponding author: ZAHIDI Hatim Amine, Department of Cardiology, IBN ROCHD Casablanca University Hospital, 8, Rue Lahcen El Arjoun Casablanca 20100, Morocco
Submission: May 08, 2023;Published: June 01, 2023

ISSN:2690-9707 Volume2 Issue4
Introduction
Acute myocarditis is an inflammatory disease of the heart that has variable clinical presentations and is caused by a range of underlying inflammatory variants [1,2]. Of newonset heart failure, 10% to 30% may be caused by cardiac inflammation and viral infection [3,4]. Systemic or local inflammatory diseases, as well as genetic predisposition are the usual risk factors [5-7]. Rheumatoid arthritis (RA) is an autoimmune disease primarily affecting the joints, but systemic involvement should never be overlooked.
Cardiovascular complications of RA are dominated by pericarditis and also include valve diseases, atrio-ventricular conduction disorders, coronary artery diseases and myocarditis [8]. Cardiac involvement in rheumatoid arthritis is a prognostic factor that marks a major turning point in the evolution of the disease and conditions its prognosis. We present a rare case of acute myocarditis in a rheumatoid arthritic young female patient.
Case presentation
A 43-year-old female patient, without any particular pathological history, was admitted in our unit for the new onset chest pain evolving over 48 hours. The chest pain started when she was sleeping and was constrictive, constant, with no radiation. In addition, she had fatigue, palpitations but denied orthopnea.
Upon physical examination, she was alert, oriented in time and place, afebrile, with a heart rate of 86bpm, a respiratory rate of 22bpm and a blood pressure of 112/77mmHg. Oxygen saturation on room air was 98%. There was no cyanosis or peripheral edema. Physical examination showed no abnormalities. An electrocardiogram was performed showing a regular sinus rhythm with a complete left bundle branch block and secondary depolarization disorders. Chest X-ray showed normal bilateral lung fields and cardiac silhouette. Initial laboratory workup showed elevated troponin level at 430 and C-reactive protein at 33mg/l. Basic metabolic panel was within normal intervals.
Figure 1:Echocardiogram of the patient showing normal ejection fraction and no significant valvular lesion.
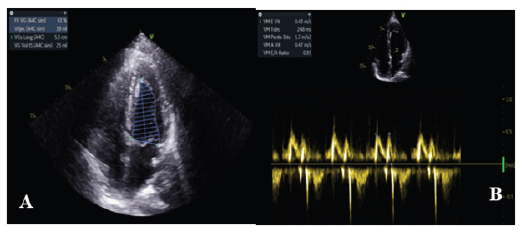
Echocardiography revealed a non-dilated left ventricle (LV end diastolic diameter (LVEDD): 27mm/m2) with septal dyskinesia related to the left bundle branch block and an ejection fraction of 56% (Simpson biplane). There was no significant valvular leak or stenosis, the right ventricle had normal function and there were no signs of pulmonary hypertension (Figure 1).
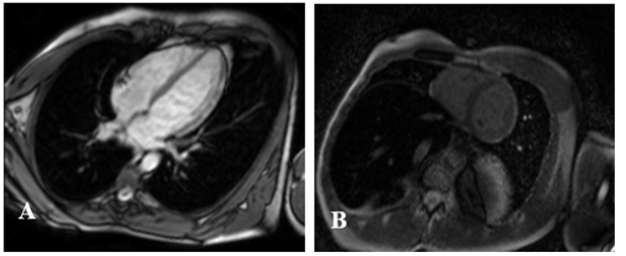
A coronary angiography was performed, which found the two coronary networks free of any lesion (Figure 2). Cardiac Magnetic Resonance (CMR) showed sub epicardial hypersignal in the anterolateral wall of the left ventricle and as evidence of edema in the T2 sequences and late sub epicardial enhancement in the same areas in the T1 sequences (Figure 3). This represented two out of three of Lake Louise’s criteria, which confirmed the diagnosis of myocarditis. After further history taking, the patient was found to have polyarthralgia in the last three months especially in the proximal inter phalangeal joints and cervical rachis. Extensive workup revealed positive rheumatoid factor and anti-citrulline antibodies. Human Immunodeficiency Virus (HIV) and hepatitis serology were negative. All these clinical and paraclinical arguments allowed to retain the diagnosis of acute myocarditis and rheumatoid polyarthritis.
Figure 2:Normal coronary angiography of the patient.
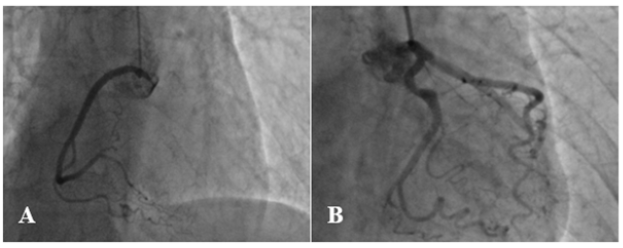
Figure 3:CMR showing late gadolinium enhancement in the antero- lateral wall.
Therapeutically, there was no need for intravenous inotropic drugs. The patient was put on beta-blockers to prevent potentially serious and fatal rhythm disturbances. Treatment for rheumatoid arthritis was started with long term steroids and subcutaneous Methothrexate. She reported an improvement in her symptoms with a regression of chest pain and the disappearance of her the dyspnea after 6 months. Transthoracic echocardiogram at 6-month follow-up showed an improvement of the ejection fraction calculated at 63%.
Discussion
Although rheumatoid arthritis is an autoimmune inflammatory disease that mainly affects the joints, the systemic involvement should not be ignored. The cardiac involvement during this pathology results in numerous manifestations that can be responsible for the alteration in her quality of life and the reduction of life expectancy. Myocardial damage during RA is generally silent; it can be due to several mechanisms including mainly micro or macro coronary artery disease, inflammation and myocardial fibrosis.
Autopsies show that cardiac involvement was present in 50% of RA patients, dominated by pericarditis; myocarditis was encountered in 4 to 30% of these patients [8]. Myocarditis remains a rare but described complication of RA, clinically manifested mainly by chest pain associated with dyspnea, but it may be discovered on autopsy following sudden death from severe rhythm disturbances.
Two histological forms of myocarditis can be observed in patients with RA: the interstitial form and the granulomatous form. The latter is more specific for RA, while the interstitial form is rather common in systemic lupus erythematosus [9]. Granulomatous lesions specific to RA can be described as yellowgray spots in the endocardial surfaces, often localized in the left ventricle. Microscopically, they consist of a central core of fibrinoid necrosis fibroblasts surrounded by a layer of palisading histiocytes, multinucleated giant cells and an outer zone of fibrous tissue with chronic inflammatory cells [9].
The endomyocardial biopsy represents the gold standard. However, the high morbidity of the procedure (estimated at about 0.5%), its low sensitivity (between 10 and 20%) and its interobserver variability do not encourage practice of this examination routinely [10]. The complications of endomyocardial biopsy have led researchers to develop new, less invasive techniques for diagnosing acute myocarditis.
Cardiac resonance is currently the reference method for the diagnosis of acute myocarditis. It allows a positive diagnosis with high sensitivity and specificity based on the Lac Louise criteria developed in 2006, and thanks to the new methods of tissue characterization, T1 and T2 mapping. T2-weighted imaging allows detection of myocardial edema using the long T2 of water molecule protons. Late post-gadolinium enhancement reflects early and late myocardial damage (necrosis and fibrosis) and is mostly irreversible. It represents an unavoidable sequence in the diagnosis of acute myocarditis [11] and is performed in T1 sequence approximately five to ten minutes after gadolinium injection. The use of corticosteroids and non-steroidal anti-inflammatory drugs has been associated with a significant risk of congestive heart failure, a risk that remains dose-dependent for corticosteroids. Methotrexate, on the other hand, remains a good therapeutic option and has been shown to reduce cardiovascular mortality when administered at a dose of 25mg per week [12]. Coronary artery disease remains the leading cause of myocardial injury worldwide and the main cause of heart failure. Acute myocarditis is a relatively rare complication of many autoimmune diseases, including rheumatoid arthritis. This diagnosis can only be made after eliminating coronary and infectious causes, which are more frequent. Once this diagnosis has been confirmed, immunosuppressive treatment can be initiated in the absence of contraindications.
In our patient’s case, the absence of cardiovascular risk factors, young age and the atypical chest pain made us think of the diagnosis of acute myocarditis. A troponin assay was performed to determine myocardial involvement, and then coronary angiography was performed to rule out coronary involvement. CMR has subsequently confirmed the diagnosis of acute myocarditis. The immunological workup and negative infection serologies were also in favor of the same diagnosis. All these clinical and biological, and imaging findings lead us to the diagnosis of acute myocarditis revealing rheumatoid polyarthritis.
Conclusion
Our case report shows a case of acute myocarditis revealing rheumatoid arthritis, an unusual situation in current practice. We also reported the role of cardiac MRI in the study of the myocardium and its importance for the confirmation of myocarditis. Immunosuppressive treatment with the classical treatment of heart failure remains the best therapeutic alternative. In our case, the early management and adequate treatment allowed a favorable evolution.
Consent for Publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images.
Competing Interests
The authors declare that they have no competing interests.
Author’s Contributions
All authors were involved in the diagnosis, management, and care of the patient. All authors contributed equally to writing the manuscript.
Funding’s
This work wasn’t supported by any funding.
Acknowledgement
No acknowledgements for this article.
References
- Lieberman EB, Hutchins GM, Herskowitz A, Rose NR, Baughman KL (1991) Clinicopathologic description of myocarditis. J Am Coll Cardiol 18(7): 1617-1626.
- Cooper LT (2009) N Engl J Med 360(15): 1526-1538.
- Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, et al. (2005) Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112(13): 1965-1970.
- Maekawa Y, Ouzounian M, Opavsky MA, Liu PP (2007) Connecting the missing link between dilated cardiomyopathy and viral myocarditis: virus, cytoskeleton, and innate immunity. Circulation 115(1): 5-8.
- Caforio AL, Keeling PJ, Zachara E, Mestroni L, Camerini F, et al. (1994) Evidence from family studies for autoimmunity in dilated cardiomyopathy. Lancet 344(8925): 773-777.
- Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, et al. (2006) Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation 114(12): 1269-1276.
- Pulerwitz TC, Cappola TP, Felker GM, Hare JM, Baughman KL, et al. (2004) Mortality in primary and secondary myocarditis. Am Heart J 147(4): 746-750.
- Cathcart ES, Spodick DH (1962) Rheumatoid heart disease: a study of the incidence and nature of cardiac lesions in rheumatoid arthritis. N Engl J Med 266: 959-964.
- Ferrans VJ, Rodríguez ER (1985) Cardiovascular lesions in collagen-vascular diseases. Heart Vessels Suppl 1: 256-261.
- Dec GW, Palacios IF, Fallon JT, Aretz HT, Mills J, et al. (1985) Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med 312(14): 885-890.
- Friedrich MG, Sechtem U, Schulz Menger J, Holmvang G, Alakija P, et al. (2009) Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 53(17): 1475-1487.
- Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, et al. (2011) Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 108(9): 1362-1370.
© 2023 Zahidi HA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






