- Submissions

Full Text
Associative Journal of Health Sciences
Regenerative Endodontics of Necrotic Immature Permanent Teeth: Scientific Integrity Review
Ana Carolina Neckel1, Júlia Vitali Dondossola1, Patrícia Maria Poli Kopper2, Josiane de Almeida3, Renan Antônio Ceretta4 and Anarela Vassen Bernardi4*
1Departamento Ciências da Saúde, Curso de Odontologia, Universidade do Extremo Sul Catarinense, Santa Catarina, Brasil
2Departamento de Odontologia Conservadora, Curso de Pós-graduação em Odontologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brasil
3Departamento de Ciências da Saúde, Curso de Odontologia, Disicplina de Endodontia, Universidade do Sul de Santa Catarina, Santa Catarina, Brasil
4Departamento de Ciências da Saúde, Curso de Odontologia, Universidade do Extremo Sul de Santa Catarina, Santa Catarina, Brasil
*Corresponding author: Anarela Vassen Bernardi, Department of Health Sciences, Dentistry Course, Universidade do Extremo Sul de Santa Catarina, Santa Catarina, Brazil
Submission: August 30, 2022;Published: September 13, 2022

ISSN:2690-9707 Volume2 Issue1
Abstract
The aim of this study was to identify a clinical protocol on regenerative endodontics based on scientific evidence for necrotic immature permanent teeth, as well as to conduct a critical evaluation of the results for modifying the clinical protocol of the Dentistry undergraduate course at the Universidade do Extremo Sul Catarinense (UNESC). The research is a qualitative, retrospective, descriptive and documental typified as literature review. The research was conducted using the following databases: PubMed and Latin American Literature and from the Caribbean on Health Sciences (Lilacs), using a combination of the following terms: Regenerative Endodontics OR Immature Tooth AND MTA. The research was concluded on 21 March 2021. The inclusion criteria were presence of the keywords, English language, publication years from 2015 to 2021 and dentistry journals with an impact factor higher than 0.001. The exclusion criteria were articles that were repeated between the databases, abstracts that did not present the researched topic and articles without available abstracts. Eleven articles have been identified and several protocols for regenerative endodontic treatment. It is concluded that the literature presents relevant requirements that provide reflections for the change of the traditional protocol hitherto performed at UNESC. Regenerative endodontics is an alternative treatment for necrotic immature teeth.
Keywords:Regenerative endodontics; Dental pulp necrosis; Stem cells
Introduction
Injuries to young permanent teeth in children and adolescents are often associated with orofacial trauma, which can occur before complete root development [1]. Damage to the periapical region can affect the cells of the apical papilla and the periodontal ligament resulting in pulp inflammation or necrosis, preventing root development [2]. Performing endodontic treatments in teeth with incomplete root formation is a significant challenge in endodontics, due to the thin dentin walls and open apex that make it difficult to instrument the canal and make an adequate apical stop [1,3].
Apexification is a treatment option aimed at inducing the formation of an apical mineralized tissue barrier by using calcium hydroxide paste [3]. This protocol has several disadvantages, such as multiple treatment visits, possibility of recontamination of the canal and increased dentin fragility, which can lead to radicular fracture [3].
In the 60’s, the first report of an alternative protocol for necrotic immature teeth, called pulp revascularization, appeared in the literature, aiming at enabling the completion of root development [2]. The aim of this work was to identify research with clinical protocols in regenerative endodontics for teeth with incomplete root formation based on scientific evidence, as well as to carry out a critical evaluation of the results to propose a modification in the clinical protocol of the Dentistry course at UNESC.
Literature Review
Concept and indications of pulp revascularization
In the literature, the terms Regenerative Endodontics, Revascularization, and Revitalization are synonymous and can be used interchangeably [4]. The first studies of Regenerative Endodontics started right after the 60s, with the publication of experimental studies in dogs by Östby [5], with the objective of evaluating how periodontal tissue reacted when the pulp was removed from the root canal. and the apical portion was filled with blood.
According to Kim et al. [4], the first revascularization work demonstrating clinical success was performed by Iwaya et al. [6] in an inferior second premolar with incomplete root formation. The removal of the infected necrotic coronary pulp was performed followed by a protocol of disinfection and maintenance of the apical pulp tissue in order to promote revascularization. The tooth responded positively to treatment and root development continued until apical closure [6].
Both the American Association of Endodontics (AAE) [7] and the European Society of Endodontics (ESE) [8] consider pulp revascularization an alternative for endodontic treatment. According to the AAE [8], regenerative endodontics is characterized by the use of biological procedures designed to physiologically replace damaged tooth structures.
Pulp revascularization consists of the use of antimicrobial agents to reduce infection, minimal instrumentation of the canal walls, associated with induction of apical bleeding to form a blood clot tightly sealed to the root canal in order to promote repair [9].
According to Palma et al. [10] regenerative endodontics aims to promote continued root development and establish a positive response to the pulp vitality test. According to Staffoli et al. [9], revascularization is indicated for teeth with necrotic pulp and, according to Lin et al. [11], treatment success depends on the size of the apical opening and the patient’s immune response.
According to the AAE [7], endodontic regeneration is recommended in teeth with pulp necrosis and incomplete rhizogenesis, with up to 2/3 of root formation. The induction of intracanal bleeding should be performed to create a clot, which will be a matrix for stem cells and growth factors in the canal from the apical papilla, so that there is the possibility of tissue regeneration [12].
The presence of viable cells in the apical papilla enables tissue regeneration due to the pluripotent capacity conferred by stem cells, that is, they can differentiate into various types of cells in the human body. One way to perform revascularization is via cell migration, which occurs naturally from chemotaxis, using pulp stem cells. There are other ways to perform revascularization, for example, from stem cell transplantation; however, this method is more complex because it requires the manipulation of cells in vitro and since it is a more expensive procedure [13-15].
Methods
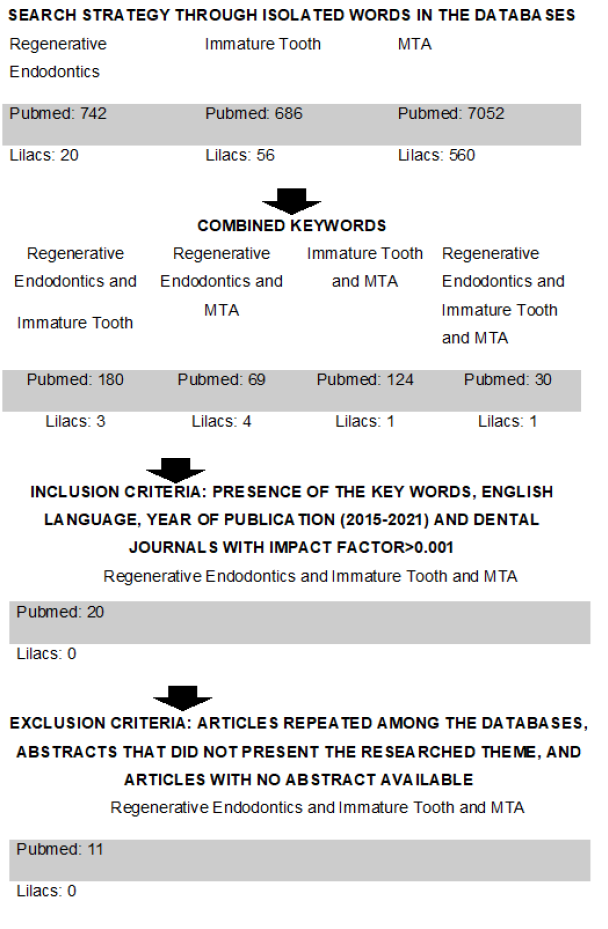
This is a qualitative, retrospective, descriptive, documentary research of the literature review type. The research was conducted using the following databases: PubMed and Latin American and Caribbean Literature on Health Sciences (Lilacs), using the combination of the following terms: Regenerative Endodontics OR Immature Tooth AND MTA. The study was completed on March 21, 2021. The inclusion criteria were presence of the keywords, English language, publication year 2015-2021, and dental journals with an impact factor greater than 0.001. The exclusion criteria were articles repeated among the databases, abstracts that did not present the researched theme, and articles with no abstract available.
As an itinerary for the computation of the articles, the following were used (Figure 1).
Figure 1:Search Strategy Flowchart. Researcher’s data, 2021.
Result
Data from the 11 articles were organized and described chronologically, considering the following variables: year of publication, title, journal, impact factor and type of study (Table 1). No article was excluded after applying the exclusion criteria.
Table 1: Characteristics of the studies according to publication year, title, journal, and impact factor.
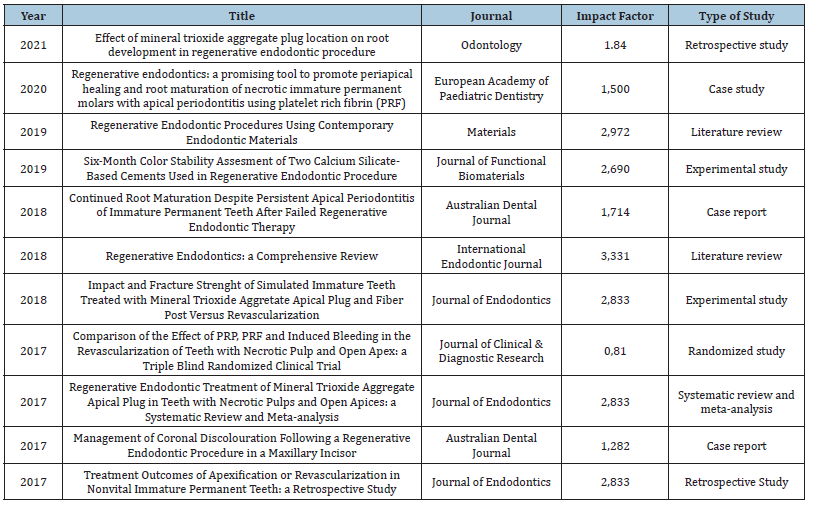
Source: Researcher’s data.
Discussion
Regenerative Endodontics represents a significant advance in the field. The idea that damaged structures can be replaced, and functionality restored (such as nociception and immune competence) in previously necrotic and infected root canal systems has been innovative [8]. Numerous cases of successful endodontic regeneration have been reported in the literature and some of these could be seen in the course of this study [4,9,11,16,17].
Pulp regeneration is an alternative treatment for teeth with incomplete rhizogenesis and pulp necrosis. When it is performed, the patient needs to receive the information about the treatment by means of an informed consent. This document should explain in detail the treatment, duration, need for follow-up, existing treatment alternatives, the allergenic potential of the antimicrobials used, and other adverse reactions. In addition, the possibilities of tooth discoloration, lack of scarring, pain and infection should be clarified to the patient [9]. The AAE [8] adds that the patient/ guardian must be cooperative to the treatment.
According to Staffoli et al. [9] and Silujjai [18], the teeth selected for pulp revascularization must be necrotic and with an open apex.
Kim et al. [4] in their study, mention that necrotic immature permanent teeth that require an intraradicular post for coronal restoration are not suitable for pulp revascularization, the best treatment being the MTA apical plug and root canal filling. Galler et al. [8] add that avulsed teeth, immediately after reimplantation, and teeth that cannot be adequately isolated, are also unsuitable for treatment.
According to Kim et al. [4], the probability of success in teeth with immature apex depends on the size of the apical opening, and the larger, the greater the possibility of vessel and stem cell growth in the root canal. In contrast, Fang et al. [19] concluded that teeth with an apical diameter smaller than 1mm achieved clinical success after pulp revascularization than teeth with an apical diameter between 0.5mm and 1mm. The authors attributed this fact to factors such as patient age, etiology of pulp necrosis, apical radiolucency, procedure performed and follow-up period.
Silujjai [18] described that teeth undergoing revascularization treatment that were unsuccessful showed signs and symptoms of apical periodontitis caused by persistent infection. According to Fouad [20], for the success of pulp revascularization to be achieved, the infection of the root canal system must be controlled.
Clinical protocol
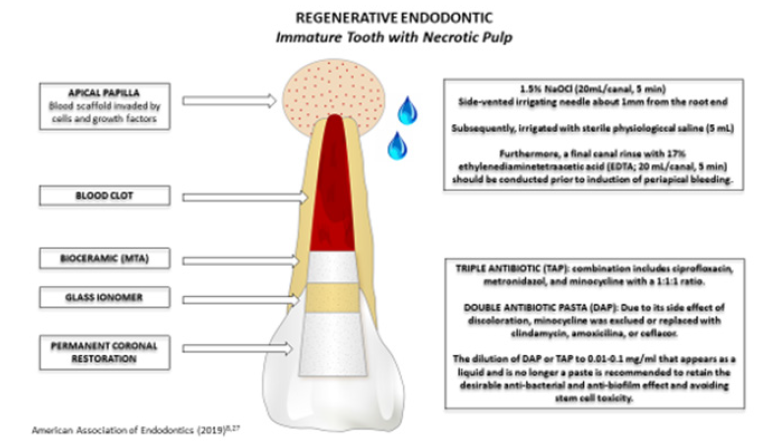
The clinical protocol for pulp revascularization is performed in two stages and comprises: local anesthesia, absolute isolation, endodontic opening, chemical-mechanical preparation, intracanal medication (first stage); bleeding/clot induction, cervical plug with tricalcium silicate cement, coronary shielding (second step) and preservation. According to the AAE [7] and ESE, the pulp revascularization protocol requires at least two visits for treatment (Figure 2).
Figure 2:Regenerative endodontic protocol immature tooth with pulp necrosis.
Endodontic opening
The authors did not present any particularities at the time of endodontic access, they perform local anesthesia, absolute isolation and access normally.
Chemical mechanical preparation
Mechanical preparation is, usually, part of the disinfection protocols, however, in cases of pulp revascularization the practice is not recommended because it reduces the resistance of the already weakened walls of the root canals, which can lead to fractures [4,8,9,11,19]. According to the ESE [7], necrotic tissue must be removed along the entire length of the canal using endodontic instruments, avoiding mechanical instrumentation of the root canal walls.
The chemical preparation uses disinfection methods that start with irrigation with 1.5% NaOCl (20mL/root canal, 5min), and for large canals this action can be enhanced through passive ultrasonic irrigation (PUI) and use XP-endo (FKG Dentaire SA, La Chaux-de- Fonds, Switzerland), followed by 5mL of saline to minimize the cytotoxic effects of NaOCl on vital tissues. Subsequently, irrigation is performed with 17% EDTA (20mL/root canal, 5min) [8.21].
Irrigation should be gentle and with a needle with a lateral opening to avoid irritation of the periapical tissues entering the canal at approximately 1 to 2mm below the root apex [7,8]. There is also the possibility of using chlorhexidine, which has antimicrobial properties and has a residual effect [21,22]. However, studies show that chlorhexidine can also have a cytotoxic effect depending on the concentration [23,24], in addition to not being able to dissolve organic tissue [25].
According to Staffoli et al. [9], NaOCl is the most used irrigating solution in regeneration and concentrations range from 1% to 6%. But current protocols, presented by the author, recommend the use of NaOCl at a concentration of 1.5% to 3% to achieve a balance between disinfection and cell protection [7]. The author indicates the use of sterile saline solution to wash all the sodium hypochlorite (NaOCl) solution, as the NaOCl solution makes it difficult for stem cells to adhere to the root canal surface. It was suggested by Kim et al. [4] that high concentrations of NaOCl and triantibiotic paste are harmful to the survival and differentiation of stem cells from the apical papilla, possibly negatively influencing root development.
It is possible to notice a variation in the concentration of the NaOCl solution in different protocols. Lin et al. [11] used the NaOCl solution at a concentration of 3.25%; in contrast, Shivashankar et al. [26] used NaOCl at 5.25%; Silujjai [18] applied concentrations of 1.5 to 2.5%; Dmello [16] used a 1% concentration. EDTA (Ethylenediamine Tetraacetic Acid) stands out as another important agent used in revascularization procedures and its main function is to remove the smear layer, a product of the instrumentation process that tends to cover dentinal tubules [4]. The demineralizing action of EDTA allows the disinfection process to be more effective through the clearance caused by dentin scrapings, debris from adjacent tissues and irrigation fluids resulting from the mechanical process [27]. In addition to the primary function, Galler et al. [7], describe that EDTA has positive effects in the regenerative procedure, through the release of growth factors by the exposed dentin matrix after demineralization, helping in secondary functions such as adhesion, migration and differentiation of stem cells from the pulp tissue. The use of EDTA in pulp revascularization is a consensus among researched authors. The AAE [8] recommends the use of 1.5% NaOCl, followed by sterile saline solution, and finally 17% EDTA.
Intracanal medication
As a complement to the disinfection of root canals, intracanal drugs are used, such as calcium hydroxide paste and triantibiotic paste [8]. Triantibiotic paste is the most used intracanal dressing, as it is an effective medication to control infection in the root canal system and to favor the healing of apical tissues [9]. It consists of a paste-like mixture of equal parts ciprofloxacin, metronidazole and minocycline with sterile saline solution [4,9]. Although biocompatible, the undiluted mixture of antibiotics has a detrimental effect on stem cell survival, the concentrations should be bactericidal, but with minimal detrimental effects on stem cell viability [9]. The paste can be inserted into the canal 1 to 2mm below the apex, with a lentulo bur or a syringe [9]. The AAE [8] indicates the non-use of minocycline in teeth that involve esthetics, using a double antibiotic paste or replacing it with another antibiotic such as amoxicillin, clindamycin or cefaclor, in order to minimize the possibility of tooth discoloration, an unwanted effect caused by most cyclins.
Triantibiotic paste is used in low concentration, from 1mg/mL to 5mg/mL in regenerative procedures [8]. The most current AAE recommendation [21] is to dilute the double or triple antibiotic paste to 0.01-0.1mg/mL in liquid to retain the desirable antibacterial effect and avoid stem cell toxicity. Calcium hydroxide paste is an alternative as an intracanal medication as it has bactericidal and bacteriostatic potential as it has a pH above 12, which hinders bacterial proliferation and tends to eliminate most species of pathogenic microorganisms susceptible to the high hydrogenic potential of Ca(OH)2 [28]. Ca(OH)2 is recommended for use when there is hypersensitivity of the patient to one of the antibiotics used in the triantibiotic paste and should be applied only in the coronal half of the root canal, given its high pH that can destroy the apical papilla cells and tissues periapical [9].
In the study by Silujjai [18], the intracanal medication used was calcium hydroxide paste or triantibiotic paste containing 250mg ciprofloxacin, 400mg metronidazole and 50mg minocycline in a ratio of 1:1:1. In the study by Lin et al. [11] calcium hydroxide paste was the intracanal medication option. In both studies, the results obtained with the medications used were satisfactory. The protocol adopted by Shivashankar et al. [26], used a triantibiotic paste containing 400mg metronidazole, 200mg ciprofloxacin and 100mg minocycline in the same 1:1:1 ratio, prepared in creamy consistency using propylene glycol and polyethylene glycol in a 1:1 ratio, the patient remained on the medication for three weeks.
Among the eleven studies analyzed, five used triantibiotic paste as intracanal medication and four used calcium hydroxide paste. It was observed that the success rate of both medications is high. However, in the study by Silujjai [18], cases in which revascularization therapy failed were treated with calcium hydroxide. After placing the intracanal medication according to the AAE protocol [8], the access cavity should be sealed with 3-4mm of temporary restorative material such as Cavit™, IRM™, glass ionomer or another temporary material. After this procedure, the patient should be instructed to return within 1 to 4 weeks.
Bleeding induction, biomaterial and coronary shielding
Staffoli et al. [9] and AAE [8] describe that if the patient has persistent symptoms and/or there are signs of infection, additional time of treatment with the same intracanal medication or change of this medication may be necessary. Galler et al. [7] add that the administration of systemic antibiotics can be considered if the patient reports general health changes, such as fever or dysphagia. To induce bleeding from the periapical tissues into the canal, local anesthesia without vasoconstrictor should be performed (so that it does not interfere with bleeding), tooth reopening, irrigation with 20mL of 17% EDTA and drying of the root canal with tips of absorbent paper [8,9]. In contrast, Galler et al. [7] mention that evidence of improvement in bleeding using anesthetic without vasoconstrictor is scarce, and the difficulty in creating the clot is more related to the patient’s sensation of pain.
With the patient anesthetized, Lin et al. [11] and Staffoli et al. [9] describe that the periapical tissues should be stimulated with an endodontic file to induce bleeding into the canal space, the blood should clot in approximately 15 minutes. The AAE recommendation for the induction of bleeding is that a pre-curved K-file be introduced into the canal, exceeding 2mm from the apical foramen, with the goal of promoting bleeding that fills the entire canal up to the level of the amelo-cemental junction [8]. After bleeding has been induced, it should be stabilized by pressing a cotton dressing soaked with sterile saline solution, positioned below the amelocemental junction in the apical direction. Thus, the objective is to create a clot inside the canal, which will serve as support for mesenchymal stem cells and growth factors, from the apical papilla, to establish themselves, promoting tissue regeneration [13].
According to Kim et al. [4], it is not always possible to induce bleeding, due to the destruction of periapical tissues. If induction cannot be achieved at the first visit after using intracanal medication, the procedure can be postponed to subsequent visits, allowing time for the periapical tissues to recover from the injury. In contrast, Staffoli et al. [9] stated that if bleeding does not occur, it may be necessary to irrigate the canal with 17% EDTA and perform over-instrumentation, preventing blood clotting. The authors added as an alternative the use of platelet-rich plasma or fibrin as a bridge, but the procedure requires a qualified professional to remove the patient’s blood and manipulate it. Shivashankar et al. [26] mentioned platelet-rich plasma (PRP) as a scaffold for the treatment of pulp revascularization, as it contains large concentrations of growth factors.
After the formation of the clot, the AAE [8] indicates, if necessary, the placement of a resorbable matrix such as CollaPlug™, Collacote™, CollaTape™ over the blood clot followed by the application of MTA, as capping material, and, on top of it, a 3-4mm layer of glass ionomer. Lin et al. [11] used an absorbable collagen dressing over the blood clot followed by the application of MTA 3mm thick above the dressing, at the limit of the cementoenamel junction, sealing the access cavity with glass ionomer cement. In the study by Silujjai [18], MTA was applied with a thickness of 2-3mm and coronal sealing with definitive restoration.
Jun et al. [29] report in their study that the apically positioned MTA is beneficial for the growth of hard tissue and root formation, but it can obliterate the root canals and prevent the growth of pulp tissue. In contrast, coronally positioned MTA is favorable for pulp tissue growth, even if root growth is limited. It should be noted that conventional MTA can cause pigmentation of the dental crown, which can also occur due to the hemoglobin present in the dentinal tubules [16]. Jun et al. [29] suggest avoiding the application of MTA above the cementoenamel junction to avoid coronary discoloration.
More recently, other bio-ceramic materials, also known as tricalcium silicate cements, have been developed as an alternative to conventional MTA. As well as MTA, these new materials have been shown to be biocompatible, being able to induce osteogenic differentiation [9].
Proservation
The success of regenerative endodontics is established when there is elimination of symptoms, evidence of bone recovery, thickening of the walls, continuation of root formation and a positive vitality test [7,8]. Shivashankar et al. [26] report that there was a gradual change in the percentage of negative to positive responses to the pulp sensitivity test during the 12-month follow-up period and that, after this period, the response to the test was positive. Lin et al. [11] used pulp sensitivity, percussion and palpation tests during case follow-up.
According to the AAE [8], the follow-up of the case must be carried out at 6, 12 and 24 months after treatment. After these periods, follow-up should become annual. Furthermore, it emphasizes that the use of computed tomography is highly recommended for follow-up. Agreeing with these follow-up periods, Galler et al. [7] recommend follow-up for 6, 12, 18 and 24 months after treatment and, after these intervals, that the follow-up starts to be carried out annually for 5 years [7].
According to Staffolim et al. [9], the follow-up protocol is performed by periodic appointments for clinical and radiographic evaluation, suggesting follow-up every 3 months. Clinical examination should not show pain on palpation and percussion and soft tissue swelling. Radiographs should be taken to observe resolution of apical radiolucency, thickening of the root walls and/or increase in root length. Sensitivity testing must also be performed. Lin et al. [11]. recommend regular follow-up at 3, 6, 12 months, and 2 years. In contrast, Shivashankar et al. [26] followed up at intervals of 3, 6, 9 and 12 months, evaluating the following criteria: absence of pain, inflammation or swelling, radiography showing the presence of periodontal ligament without alterations.
Conclusion
It is concluded that the literature presents relevant subsidies that provide reflections for the change of the traditional protocol until then executed at UNESC. Regenerative endodontics is an alternative treatment for necrotic immature teeth.
References
- Cehreli ZC, Sara S, Uysal S, Turgut MD (2010) MTA apical plugs in the treatment of traumatized immature teeth with large periapical lesions. Dent Traumatol 27(1): 59-62.
- Nagata JY, Gomes BPFA, Lima TFR, Murakami LS, Faria DE, et al. (2014) Traumatized immature teeth treated with 2 protocols of pulp revascularization. J Endod 40(5): 606-612.
- Nosrat A, Seifi A, Asgary S (2011) Regenerative endodontic treatment (Revascularization) for necrotic immature permanent molars: A review and report of two cases with a new biomaterial. J Endod 37(4): 562-567.
- Kim SG, Malek M, Sigurdsson A, Lin LM, Kahler B (2018) Regenerative endodontics: a comprehensive review. Int Endod J 51(12): 1367-1388.
- Östby BN (1961) The role of the blood clot in endodontic therapy an experimental histologic study. Acta Odontol Scand 19(3-4): 323-353.
- Iwaya S, Ikawa M, Kubota M (2001) Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol 17(4): 185-187.
- American Association of Endodontists (AAE) (2020) AAE clinical considerations for a regenerative procedure.
- Galler KM, Krastl G, Simon S, Van Gorp G, Meschi N, et al. (2016) European society of endodontology position statement: revitalization procedures. Int Endod J 49(8): 717-723.
- Staffoli S, Plotino G, Torrijos BGN, Grande NM, Bossù M, et al. (2019) Regenerative endodontic procedures using contemporary endodontic materials. Materials 12(6): 908-915.
- Palma P, Marques JA, Falacho RI, Correia E, Vinagre A, et al. (2019) Six-month color stability assessment of two calcium silicate-based cements used in regenerative endodontic procedures. J Funct Biomater 10(1): 14-20.
- Lin LM, Kim SG, Martin G, Kahler B (2018) Continued root maturation despite persistent apical periodontitis of immature permanent teeth after failed regenerative endodontic therapy. Aust Endod J 44(3): 292-299.
- Lovelace TW, Henry MA, Hargreaves KM, Diogenes A (2011) Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod 37(2): 133-138.
- Kim SG, Zheng Y, Zhou J, Chen M, Embree MC, et al. (2013) Dentin and dental pulp regeneration by the patient's endogenous cells. Endod Topics 28(1): 106-117.
- Potdar PD, Jethmalani YD (2015) Human dental pulp stem cells: Applications in future regenerative medicine. World J Stem Cells 7(5): 839-851.
- Xiao L, Nasu M (2014) From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Cloning 7: 89-99.
- D'Mello G, Moloney L (2017) Management of coronal discolouration following a regenerative endodontic procedure in a maxillary incisor. Aust Dent J 62(1): 111-116.
- Yoshpe M, Kaufman AY, Lin S, Ashkenazi M (2021) Regenerative endodontics: a promising tool to promote periapical healing and root maturation of necrotic immature permanent molars with apical periodontitis using platelet-rich fibrin (PRF). Eur Arch Paediatr Dent 22(3): 527-534.
- Silujjai J, Linsuwanont P (2017) Treatment Outcomes of apexification or revascularization in nonvital immature permanent teeth: A retrospective study. J Endod 43(2): 238-245.
- Fang Y, Wfang X, Zhu J, Su C, Yang Y, et al. (2018) Influence of apical diameter on the outcome of regenerative endodontic treatment in teeth with pulp necrosis: A review. J Endod 44(3): 414-431.
- Fouad AF (2017) Microbial factors and antimicrobial strategies in dental pulp regeneration. J Endod 43(9S): 46-50.
- American Association of Endodontists (AAE) (2019) Infection Control in Regenerative Endodontic Procedures.
- Greenstein G, Berman C, Jaffin R (1986) Chlorhexidine: an adjunct to periodontal therapy. J Periodontol 57(6): 370-377.
- Bonacorsi C, Raddi MS, Carlos IZ (2004) Cytotoxicity of chlorhexidine digluconate to murine macrophages and its effect on hydrogen peroxide and nitric oxide induction. Braz J Med Biol Res 37(2): 207-212.
- Vouzara T, Koulaouzidou E, Ziouti F, Economides N (2016) Combined and independent cytotoxicity of sodium hypochlorite, ethylenediaminetetraacetic acid and chlorhexidine. Int Endod J 49(8): 764-773.
- Naenni N, Thoma K, Zehnder M (2004) Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod 30(11): 785-787.
- Shivashankar VY, Johns DA, Maroli RK, Sekar M, Chandrasekaran R, et al. (2017) Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: A triple blind randomized clinical trial. J Clin Diagn Res 11(6): ZC34-ZC39.
- Jafarzadeh H, Shalavi S, Mohammadi Z (2013) Ethylenediaminetetraacetic acid in endodontics. Eur J Dent 7(5): S135-S142.
- Estrela C, Sydney GB, Bammann LL, Felippe Júnior O (1995) Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J 6(2): 85-90.
- Jun JH, Chun KA, Kum KY, Lee WC, Shon WJ, et al. (2021) Effect of mineral trioxide aggregate plug location on root development in regenerative endodontic procedure. Odontology 109(2): 411-421.
© 2022 Anarela Vassen Bernardi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






