- Submissions

Full Text
Associative Journal of Health Sciences
The Role of Vitamin a in the Control of Hyperglycemia in Type 2 Diabetes Mellitus: A Narrative Review
Desiree R Daniega*
HNF 221- Nutritional Biochemistry, University of the Philippines, Philippines
*Corresponding author: Desiree R Daniega, HNF 221- Nutritional Biochemistry, University of the Philippines, Philippines
Submission: July 13, 2022;Published: July 21, 2022

ISSN:2690-9707 Volume1 Issue5
Abstract
Objective: Diabetes Mellitus, specifically Type 2 Diabetes Mellitus which accounts for almost 90% of all
diabetes, has emerged as one of the biggest pandemics of modern times. Investigations are underway,
with different areas of focus due to the disease’ heterogeneity. This review paper will go through some
research which has been done to illustrate the role of Vitamin A in the control of hyperglycemia in T2DM.
Methods: An online Literature search was done using electronic databases from MedLine, PubMed,
Google Scholar and ResearchGate published mostly in the years 2014-2019.
Result: Retinol binding protein levels especially of RBP4 was found to be positively associated with
insulin resistance which was elucidated by the mechanism of low glucose uptake in skeletal muscles
and enhanced gluconeogenesis in the liver of mice. Furthermore, the antioxidant property of Vitamin
A was also given focus due to the fact that hyperglycemia in T2DM result to oxidative stress because of
production of reactive oxygen species (ROS). Another novel mechanism of Vitamin A’s ability to control
hyperglycemia in T2DM as demonstrated by retinol supplementation in animal studies’ is the suppression
of retinoid-x-receptor resulting to decreased adipogenesis, with consequent improved insulin sensitivity.
The other mechanisms include the following:
a) low Vitamin A level demonstrated diminished islet cells due to induced cellular stressmediated
apoptosis and reducing steatoryl-CoA desaturase 1-mediated oleic acid (SCD1) synthesis.
b) vitamin A showed slight increase in GLUT4 expression causing increase in glucose uptake in
skeletal muscles and adipocytes.
Conclusion: The different biochemical bases of how Vitamin A level affects insulin sensitivity/resistance
through its antioxidant property, expression of GLUT4 transport protein for enhanced muscle and adipose
glucose uptake, and decreased adipogenesis/lipogenesis provide strong evidence on its important role in
the control of hyperglycemic state in T2DM.
Keywords:Diabetes mellitus; Metabolic disease; Hyperglycemia; Dyslipidemia; Nutrition; Vitamins C; Vitamin A
Abbreviations: AGE: Advanced Glycation End-product; CHOP, C/EBP: Homologous Protein; CVD: Cardiovascular Disease; IL: Interleukin; IRS: Insulin Receptor Substrate; OXPHOS: Oxidative Phosphorylation; PERK: Protein Kinase R-Like Endoplasmic Reticulum Kinase; PKC: Protein Kinase C; ROS: Reactive Oxygen Species; SOCS: Suppressor of Cytokine Signaling; SOD: Superoxide Dismutase; T2DM: Type 2 Diabetes Mellitus; TNF: Tumor Necrosis Factor.
Introduction
Background
Diabetes mellitus has already been a metabolic disease of concern the past decade. In 2016, World Health Organization identified Diabetes Mellitus as the 8th leading cause of death globally. A disorder affecting the metabolism of proteins, fat and carbohydrates, it is a chronic heterogenous disorder affecting the β-cell of endocrine pancreatic gland characterized by hyperglycemia, dyslipidemia, insulin resistance, impaired insulin secretion, and the activation of pro-inflammatory mediators. Type 2, which is the most common type of the disease is due to the pancreatic β-cells’ inefficiency to produce or respond to insulin, an important glucose regulating hormone [1]. The number of people affected with the disease has been constantly increasing from 108 million in 1980 to 422 million in 2014 globally. This indicates a rise in prevalence of diabetes from 4.7% in 1980 to 8.5% in 2014, according to WHO. In the Philippines, the national prevalence of diabetes in the year 2008 was 7.2%, according to a study done by Jimeno et al. [2]. The recent National Nutrition Survey (NNS) of the Food and Nutrition Research Institute (FNRI) 2019, indicated a prevalence of pre-diabetes and diabetes combined at approximately 16.1%, with one out of every six Filipinos diagnosed with either pre-diabetes or diabetes. Moreover, local study among people newly diagnosed with diabetes in Manila indicated the following proportions of having complications due to progression of the disease: about 20% had peripheral neuropathy, 42% had proteinuria, and 2% had diabetic retinopathy [2]. These data, clearly shows that public health efforts to screen for pre-diabetes and diabetes have to be more aggressive and focused, and prevention strategies are necessary and needs to be strengthened to reduce the burden of diabetes and its complications in the Philippines.
One of the mechanisms being proposed in the onset and progression of T2DM is the role of reactive oxygen species, thus giving the antioxidants prime focus in the possibility of using them as interventions. Among the vitamins studied extensively were Vitamins C and E, ascribing a protective function in diabetes manifested in controlling ROS production, however, still with conflicting evidence (Chertow, 2004; Dembinska- Kiec,2008). Vitamin A on the other hand, despite having the highest antioxidant potential among all known vitamins, is still on its infancy phase in its role in the disease’ onset and progression. An unpublished data by the DOST-FNRI based on a secondary analysis done of the 8th NNS Clinical and Health Component, 2013 showed the following: the odds of having high fasting blood glucose concentrations among adults over 20 years of age was high for those who had high serum retinol concentrations (RR=1.94, p-0.038), providing a basis for supporting the possibility of Vitamin A metabolism as a significant influence in the development of T2DM in the Philippines. In animal model study, Shirai et al. (2016) further showed serum retinol binding protein (RBP4) being significantly higher in Goto- Kakizaki (GK) rats with diabetes compared to fat-induced obesity Wistar rats, indicating that elevation of serum RBP4 is correlated with diabetes rather than obesity. Serum retinol binding protein (RBP4) also correlates with hepatocyte triglyceride levels in both animals and humans and is associated with increased de novo lipogenesis (Blaner, 1989; Stefan et al., 2007). Furthermore, RBP4 induced inflammation in adipose tissue stimulating lipolysis, which leads to enhanced free fatty acids (FFA) and elevated triglyceride accumulation in the liver [3] was a significant finding as well.
A significant data show that deficiencies in Vitamin A have been noted in the Asian developing countries and in lower income groups in the United States, where there is an increasing trend in developing T2DM [4]. With these scenarios, the review paper will give light to the hypothesis that Vitamin A level has a significant role in the control of hyperglycemia in T2DM as illustrated biochemically.
Significance of the study
Diabetes Mellitus, specifically T2DM which accounts for almost 90% of all diabetes, has emerged as one of the biggest pandemics of modern times due to difficulty in its control and management. A lot of studies are underway, with different areas of focus due to the disease’ heterogeneity. The role of Vitamins has recently become interesting due to the inflammatory nature of diabetes, contributing to the oxidative insult and deficient cellular antioxidant status of pancreatic β-cells [5,6]. With Vitamin A having the highest potential among all vitamins on antioxidant property, and the possible relevant role of Vitamin A status in the control of hyperglycemia in T2DM which can as well be associated with the risk of diabetes development in the low- and middle-income countries like the Philippines, this narrative review can contribute to the pool of evidences which can be used for higher epidemiological and even clinical studies in the future.
Objectives
General: To determine the role of Vitamin A status in the control of hyperglycemia in T2DM.
Specific: To illustrate how Vitamin A metabolism is related in the disease development of T2DM.
To provide possible biochemical basis/es in the control of hyperglycemia through Vitamin A status.
Scope and limitation
The review covers relevant studies, epidemiological both observational and analytical and experimental covering animal and few clinical studies exclusively of Vitamin A and T2DM. Few of the limitations include use of secondary local unpublished data and exclusion of combined Vitamins studies’ (eg: Vitamins A, C and E) effects on T2DM hyperglycemic control.
Methods
An online Literature search was done using electronic databases mostly from MedLine, PubMed, Google Scholar and ResearchGate. Included articles are local and international review studies and experimental (mostly 2014-2019), and professionally authored books on Nutrition, Biochemistry and Endocrinology. To simplify search, it was narrowed down to Vitamin A and Type 2 Diabetes Mellitus; Mechanisms of Vitamin A in hyperglycemia control and Vitamin A status on T2DM.
Review of Related Literature
Vitamin A metabolism
Vitamin A refer to a group of compounds, known as retinoids which include retinol, retinal, retinoic acid, and retinyl ester, as well as synthetic analogues, that possess the biological activity of all-trans retinol [7]. From animal sources, Vitamin A comes as an ester, retinyl palmitate which is pale yellow crystalline and lipid soluble solid. On the other hand, carotenoids like β -carotene, pro-vitamin A are found in plants, in the absence of retinol. Both retinyl esters and carotenoids when absorbed in the intestinal mucosa are converted to retinol and stored in the liver. Retinol can either be converted to retinal which is important for vision or oxidized to form retinoic acid (RA) which is involved in controlling the expression of multiple genes on the other hand [7,8]. Retinol is transported in bloodstream by retinol binding protein (RBP). Interaction of RBP4 with two receptors, the Toll-like receptor 4 (TLR4) and the transmembrane pore stimulated retinoic acid gene6 (STRA6) which transports vitamin A bidirectional between extra- and intracellular compartment happens. These lead to the activation of c-Jun N-terminal protein kinase (JNK) pathways and JAK2/STAT5 cascade, respectively. Intracellularly, retinol binds to the cellular retinol binding protein (cRBP), and retinoid acid binds to cellular retinoic acid binding protein (cRABP). These bound retinoid particles have specific nuclear receptors (retinotic acid receptor (RAR) and retinoid X receptor (RXR)) which contain DNA- binding domains. Altered expression of binding proteins (BP) affects retinoid function, one of which is possibly impaired pancreas development, resulting in abnormal glucose and energy metabolism [9,10].
Vitamin A and T2DM pathogenesis
The egregious-11 in the pathogenesis of T2DM include some of the following characteristics: peripheral insulin resistance, dysfunctional regulation of hepatic glucose production, and impaired beta cell activity that contributes to hyperglycemia [11]. The production of reactive oxygen species (ROS) is further enhanced by the persistent hyperglycemic state. Evidence have shown that the presence of ROS result to oxidative stress, causing further inflammatory reaction, contributing to persistent hyperglycemia and insulin resistance. Conventional therapeutic approaches in the management of diabetes have little or even no effect in addressing the oxidative status of the cell. The primary purpose of present-day medical intervention involves control of hyperglycemia, which over the years becomes ineffective. This is due to inability of an insulin treatment by itself, to inhibit protein glycation, which further overwhelms the redox system [12,13]. Literature has established the significant relationship between insulin resistance and oxidative stress, thus giving recognition on the role of antioxidants in the control of diabetes [13].
Retinoids, particularly retinoic acid (RA), have displayed
promising results in vitro, focusing on its use in islet health,
repair, and replication [14]. Chien et al. [15] mentioned that
several experimental studies have also shown that women with
deficient vitamin A during pregnancy affects the child which leads
to decrease of β-cell functions. The two mechanisms proposed are:
a) reduction in the fetal β-cell replication
b) impairment of glucose-stimulated insulin secretion.
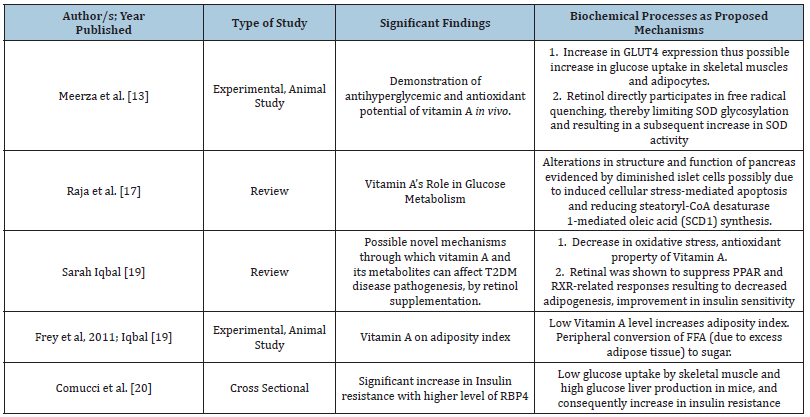
In addition, it was found that a derivative of vitamin A, retinoic acid (RA), may cause increased insulin sensitivity. On the other hand, retinoids have been discovered to possess an essential antioxidant function, while retinol binding protein (RBP) negatively affect insulin sensitivity [10]. RBP, based on some studies, is considered to be strongly associated with insulin resistance which for researchers has a strong bearing in identifying drug target in type 2 diabetes. Specifically, an increased level of retinol binding protein, particularly of RBP4, results to low glucose uptake by skeletal muscle and high glucose liver production in mice, and consequently increase in insulin resistance [16].
The several mentioned processes are previously well documented in the disease development of T2DM, which include the following: insulin resistance brought about by several mechanisms, impairment of glucose stimulated insulin secretion due to decreased pancreatic β cell function and enhanced cellular inflammation due to ROS.
Vitamin A levels and the control of hyperglycemia
An animal study published in 2018 by Raja et al. [17] resulted in plasma insulin, glucagon and C peptide levels decrease with additional perturbed overall cellular metabolism after feeding rodents with vitamin A deficient diet. Investigations further demonstrated an altered structure and function of pancreas evidenced by diminished islet cells possibly due to induced cellular stress-mediated apoptosis and reducing steatoryl- CoA desaturase 1-mediated oleic acid (SCD1) synthesis. Stearoyl coenzyme A desaturase 1 is an important enzyme to protect from leptin deficiency-induced diabetes, unsaturated fat deficiency in diet and palmitate-induced lipotoxic insults in muscle and pancreatic β-cells.
Furthermore, low Vitamin A level causes major reductions of intracellular binding protein Crbp1 and Cyp26a1, the retinoic acid metabolizing enzyme. The presence of pancreatic islet cells’ sizes reduction with the associated abnormal endocrine functions, are similar to the phenotype found in advanced type 2 diabetes [18].
The adiposity and hyperglycemia in T2DM have been a significant area of interest due to the propensity of peripheral conversion of excess fats to sugar. Most of the animal trials focused on the relation of retinol and adipose biology which yielded a strong association between adiposity and T2DM risk. A specific study reported a significant weight loss upon supplementation with high levels of dietary vitamin A (129mg/kg) in obese rats, compared with a control group receiving lower levels of dietary vitamin A (2.6mg/kg). On the other hand, a diet deficient in vitamin A (no detectable vitamin A levels in circulation) led to an increase in adiposity in mice. Therefore, this increase in adiposity is tantamount to an increased glycemic index, which is a significant risk for T2DM development (Frey et al, 2011); [19].
Meerza et al. [13] through an animal study reported that improvement in glucose flux was further confirmed by assaying changes in glucose permeability of tissues. GLUT4 is the primary glucose transporter responsible for glucose uptake in skeletal muscles and adipocytes. It is downregulated in diabetes, contributing to peripheral insulin resistance. After vitamin A treatment in diabetes-induced female albino mice, GLUT4 expression levels were assayed by sandwich ELISA in adipocytes and skeletal muscles. A slight increase in GLUT4 expression at the membrane was observed which may in turn cause possible increase in glucose uptake in skeletal muscles and adipocytes (Table 1) [13,17,19,20].
Table 1: Summary of findings based on some studies.
Summary of Results and Discussion
Understanding vitamin A metabolism vis, a vis its role in the control of hyperglycemia of T2DM is essential. Retinol binding protein levels especially of RBP4 was found to be positively associated with insulin resistance which was elucidated by the mechanism of low glucose uptake in skeletal muscles and enhanced gluconeogenesis in the liver of mice. Furthermore, the antioxidant property of Vitamin A was also given focus due to the fact that hyperglycemia in T2DM result to oxidative stress because of production of reactive oxygen species (ROS). This scenario aggravates insulin resistance; thus, Vitamin A’s antioxidant action has an interesting role in the control of ROS production and ultimately oxidative stress, thus indirectly affecting the hyperglycemic state of T2DM. Another novel mechanism of Vitamin A’s ability to control hyperglycemia in T2DM as demonstrated by retinol supplementation in animal studies’ suppression of retinoid-x- receptor resulting to decreased adipogenesis, with consequent improved insulin sensitivity. People with T2DM especially in the low- and middle-income countries also showed Vitamin A deficiency and further contributes to insulin resistance due to increased adiposity. Vitamin A supplementation thus improved the insulin sensitivity and decreased adiposity level, which helps in the control of hyperglycemia [21,22]
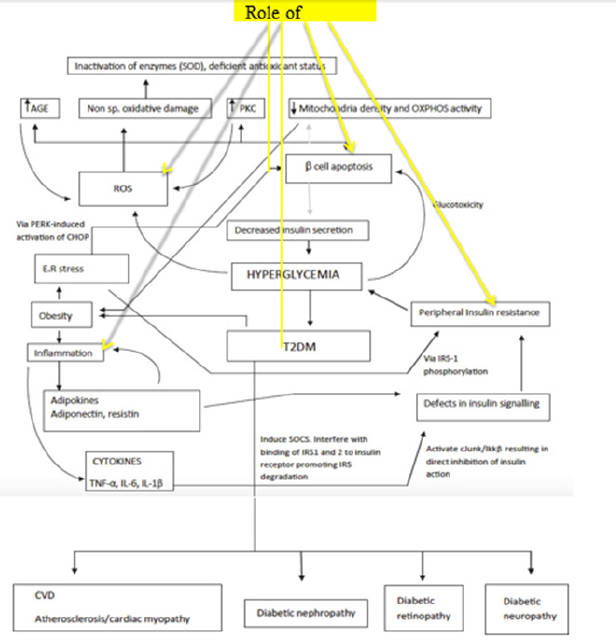
The figure below summarizes how hyperglycemia brought
about by decreased insulin secretion in T2DM becomes the starting
point of aberrations in other systems and biological processes
(Figure 1). That without controlling the hyperglycemic state,
several systemic complications develop. The role of Vitamin A in
this diagram is illustrated in four areas, namely:
1) Antioxidant property by controlling the generation of ROS.
2) Its significant contribution in inflammation as well
3) Decreased pancreatic cell damage
4) Improvement in peripheral insulin resistance.
Figure 1:Pathophysiology of T2DM development. A network of events is responsible for the establishment and progression of T2DM leading to the development of various diabetic complications. Black lines denote increase in activity; gray lines denote a diminished action. Together with few of the proposed processes positively or negatively affected by Vitamin A levels for the glycemic control in T2DM [19].
The other mechanisms which were shown in several review
articles and animal studies include the following:
a) Investigations of low Vitamin A level demonstrated an altered
structure and function of pancreas evidenced by diminished
islet cells possibly due to induced cellular stress-mediated
apoptosis and reducing steatoryl-CoA desaturase 1- mediated
oleic acid (SCD1) synthesis. Stearoyl coenzyme A desaturase
1 is an important enzyme to protect from leptin deficiencyinduced
diabetes, unsaturated fat deficiency in diet and
palmitate-induced lipotoxic insults in muscle and pancreatic
β-cells
b) Vitamin A treatment in diabetes-induce was which may in
turn cause possible increase in glucose uptake in skeletal
muscles and adipocytes, thus contributing to the control of
hyperglycemia in T2DM.
Conclusion and Recommendations
The review gave important mechanisms how Vitamin A affects glycemic control in T2DM. The link between Vitamin A level, transport system to and from the cellular milieu its metabolism, its antioxidant property and the biochemical processes in the uptake of glucose, insulin sensitivity/ resistance, adiposity/ fat accumulation are few of the documented mechanisms. Thus, Vitamin A level has an important role in the control of hyperglycemic state in T2DM.
Recommendations include more similar experimental studies to be conducted using animals with induced T2DM to gain more value and generalizability of results. Clinical or human studies can already be considered for some of the results gathered, like the correlation of biomarkers, particularly RBP4 level with glycated hemoglobin (HBa1C) which may reflect the gluconeogenesis activity of the liver among T2DM. The result maybe a benchmark in the holistic management and control of diabetes.
References
- World Health Organization (2018) Diabetes.
- Jimeno CA, Kho SA, Matawaran BJ, Durante CA, Jasul GV, et al. (2015) Prevalence of diabetes mellitus and pre-diabetes in the Philippines: A sub-study of the 7th National Nutrition and Health Survey (2008). Philippine Journal of Internal Medicine 53(2): 1-8.
- Saeed A, Dullaart RPF, Schreuderet TCMA, Blokzijlal H, Faber KN, et al. (2017) Disturbed vitamin A metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 10(1): 29.
- (2009) Low-income Americans and racial and ethnic minorities experience disproportionately higher rates of disease. US Med.
- Halliwell B (1997) Antioxidants and human disease: a general introduction. Nutrition Rev 55 (1 Pt 2): S49-52.
- Colak E, Singh NM, Stankovic S, Dordevic PB, Srekovic VD, et al. (2006) The effect of hyperglycemia on the values of antioxidative parameters in type 2 diabetic patients with cardiovascular complications. Jugoslov Med Biochem 25(2): 173-179.
- Gropper SS, Groff JL, Hunt SM (2009) The fat-soluble vitamins. In: Advanced Nutrition and Human Metabolism. West Publishing Company, Minneapolis, Minnesota, USA, pp: 273-400.
- Ross AC (1999) Vitamin A. In: Shils M, Olson JA, Shike M, Ross AC (Eds.), Modern nutrition in health and disease, (9th edn), Williams & Wilkins, Baltimore, Maryland, USA, pp: 305-313.
- Napoli JL (2017) Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol Ther 173: 19-33.
- Karolina G, Aleksandra D, Natalia J, Ewa OB (2018) Considering the role of vitamin a in glucose metabolism. J Endocrinol Diab 5(3): 1-4.
- Kahn SE, Porte D (1997) The pathophysiology of type II (noninsulin dependent) diabetes mellitus: implications for treatment. In: Porte D, Sherwin RS (Eds.), Diabetes mellitus, (5th edn), Stamford, Connecticut, USA, pp: 487-512.
- Zobali F, Avci A, Canbolat O, Karasu C (2002) Effects of vitamin A and insulin on the antioxidative state of diabetic rat heart: a comparison study with combination treatment. Cell Biochem Funct 20(2): 75-80.
- Meerza D, Iqbal S, Zaheer S, Naseem I (2016) Retinoids have therapeutic action in type 2 diabetes. Nutrition J 32(7-8): 898-903.
- Tsuji N, Ninov N, Delawary M, Osman S, Roh AS, et al. (2014) Whole organism high content screening identifies stimulators of pancreatic beta-cell proliferation. PLoS One 9(8): e104112.
- Chien CY, Lee HS, Cho CH, Lin KI, Tosh D, et al. (2016) Maternal vitamin A deficiency during pregnancy affects vascularized islet development. J Nutr Biochem. 36: 51-59.
- Gliniak CM, Brown JM, Noy N (2017) The retinol-binding protein receptor STRA6 regulates diurnal insulin responses. J Biol Chem 292(36): 15080-15093.
- Raja Gopal Reddy M, Mullapudi Venkata S, Putcha UK, Jeyakumar SM (2018) Vitamin A deficiency induces endoplasmic reticulum stress and apoptosis in pancreatic islet cells: Implications of stearoyl- CoA desaturase 1-mediated oleic acid synthesis. Exp Cell Res 364(1): 104-112.
- Trasino SE, Benoit YD, Gudas LJ (2015) Vitamin A deficiency causes hyperglycemia and loss of pancreatic β-cell mass. J Biol Chem 290(3): 1456-1473.
- Iqbal S, Naseem I (2015) Role of vitamin A in type 2 diabetes mellitus biology: Effects of intervention therapy in a deficient state. Nutrition J 31(7-8): 901-907.
- Comucci EB, Vasques ACJ, Geloneze B, Calixto AR, Pareja JC, et al. (2014) Serum levels of retinol binding protein 4 in women with different levels of adiposity and glucose tolerance. Arq Bras Endocrinol Metab 58(7): 709-714.
- DOST-FNRI (2019) E-National Nutrition Survey.
- Tomomi S, Shichi Y, Sato M, Tanioka Y, Furusho T, et al. (2016) High dietary fat-induced obesity in Wistar rats and type 2 diabetes in nonobese goto-kakizaki rats differentially affect retinol binding protein 4 expression and vitamin A metabolism. Nutr Res 36(3): 262-270.
© 2022 Desiree R Daniega. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






