- Submissions

Full Text
Associative Journal of Health Sciences
Study of Effects a New Resuspending Solution on Lipoperoxidation, Catalase Activity and Red Blood Cell Peroxidation Resistance in Donor Blood Components
Andrey Belousov1,2,4*, Ekateryna Belousova1,4, Elena Malygon2,4, Vadim Yavorskiy2,4, Militina Anoshyna3, Maryna Yagovdik3 and Tetiana Kalynychenko3
1Laboratory of Applied Nanotechnology of Belousov, Ukraine
2Kharkov Medical Academy of Postgraduate Education, Ukraine
3State Institution, Institute of Hematology and Transfusiology of the National Academy of Medical Sciences of Ukraine
4Kharkov Regional Center of Blood Service, Ukraine
*Corresponding author: Andrey Belousov, Laboratory of Applied Nanotechnology of Belousov, Ukraine
Submission: October 12, 2021;Published: December 10, 2021

ISSN:2690-9707 Volume1 Issue4
Abstract
The problem of increasing the functional activity preservation of red blood cells is priority for the Blood Service and clinical transfusiology. Taking into account the previously discovered unique properties of magnetite nanoparticles, namely: non-specific modulation of metabolic processes, strengthening of adaptive mechanisms of cellular organelles, acceleration of reparative processes at the level of membranes and macromolecules, for the first time an attempt was made to use the upgraded saline solution of sodium chloride (Nanotechnologically Upgraded Solution - NUS) as a resuspending of Erythrocyte-Containing Components (ECC). Objective: to improve the preservation of red blood cells containing donor blood components using NUS for resuspending. To achieve this goal, the first stage of the study examined the effect of a New Resuspending Solution (NUS) on lipoperoxidation, catalase activity, and red blood cell peroxidation resistance in donor blood components containing red blood cells. Object of research: Red blood cells from preservation blood of donors, which are intended for component transfusion therapy. As a result of the study, the advantages of using NUS were established, namely: a significant decrease in the levels of primary and secondary lipid peroxidation products in the first 15 days of observation, an increase in catalase activity and resistance of red blood cells to peroxidation in the first stages of the study, inhibition of the shear reaction with neutral lipid peroxidation in the late stages of observation.
Keywords: Nanomodified resuspending solution; Oxidative homeostasis; Peroxide resistance of red blood cells; Components containing red blood cells; Storage
Actuality
Extending the shelf life of blood taken from a donor is a problematic area in the field
of industrial transfusiology, which is currently receiving great attention. The global trend
towards an increase in the shortage of donor personnel encourages the development of new
technologies to provide a reserve supply of donor blood in case of extreme situations, as well
as the effective use of auto-blood blood for planned operations. Modernization of canning
and storage technologies should allow for more free management of these resources. Thus,
it was found that extending the shelf life of blood by 1 Week reduces the volume of collected
blood by 3-8%, reduces its deficiency associated with the shelf life [1,2]. In addition, it is known that the effectiveness of blood transfusion therapy largely
depends on the use of effective hemoconservants and solutions for
resuspending. Therefore, the problem of improving methods and
means of preserving blood and its components does not lose its
relevance.
One of the most important components preservation of blood
is red blood cells, the transfusion of which ensures efficient
transportation of oxygen in the patient’s body. In this regard,
it should be noted that substances that are introduced into
preservative solutions allow maintaining the metabolism of red
blood cells for a long time at a level close to the physiological
norm. Additional solutions, depending on their purpose, serve
as an additional support for the functional usefulness of blood
cell components during storage and/or are used to bring the
hematocrit of the transfusion medium to physiological parameters
by resuspending cells. The point of developing new forms of
resuspending solutions is to ensure long-term support of metabolic
processes in cells at a level as close as possible to the physiological
level. Therefore, improving the means of maintaining the functional
activity of red blood cells during their storage is the primary task of
industrial transfusiology and scientists working in the field of cell
physiology.
Separately, it should be noted that in the last decade, with the
emergence of a new promising direction of nanobiotechnologies,
there has been a significant increase in both the production of
nanomaterials and the expansion of their application areas. Studying
the structures and functions of natural nanoconstructions that exist
in a living cell is a necessary step for creating nanobiopreparations.
As in the rest of the world, a number of medical nanotechnological
products appeared in Ukraine at the end of the twentieth century
(Andrey Belousov, 1998). Among them: the ICNB (nanobiocorrector
for intravenous), the magnet-controlled sorbent (MCS-B brand),
and the biologically active additive (Micromage-B brand) [3-7].
These drugs are based on magnetite (Fe3O4) Nanoparticles (NPs)
ranging in size from 6 to 12nm, which are currently classified as
priority artificial NPs for legitimate use [8-13]. An important
advantage of this material is its highly specific (selective) sorption
properties, which creates prerequisites for the occurrence of
indirect sanogenetic effects during therapy.
So, the principle of therapeutic action of these nanotechnological
drugs is the positive effect of the adsorption process and the
constant magnetic field surrounding the magnetite NPs on cellular
and subcellular structures, as well as in changing the orientation
and mobility of hydrogen nuclear spins in the water molecule.
The point of application is the surface proteins of cell membranes.
Magnetite NPs alter the composition of protein molecules and the
polarization structure of the cellular microenvironment, actively
affecting the transport of substances into the cell [14-16]. A unique
property of these drugs is non-specific modulation of metabolic
processes. Thus, these NPs cause an increase in adaptive-adaptive
potential mechanisms and capabilities of cellular organelles,
accelerate reparative processes at the level of membranes and
macromolecules [17,18].
It was also found that these magnetite NPs act as a modulating
factor for such phenomena as metabolic processes in white blood
cells, the work of the enzyme link of the antioxidant system in
red blood cells of healthy and sick people [19]. All of the above
determined the purpose and objectives of this study, which is
related to the development of new forms of resuspending solutions
for red blood cells. Objective: to improve the preservation of red
blood cells containing components of donated blood using a
Nanotechnologically Upgraded Solution (NUS) for resuspending.
To achieve this goal, at the first stage of research, the effect of a
New Resuspending Solution (NUS) on the indicators of oxidative
homeostasis and peroxide resistance of red blood cells in red blood
cell-containing components of donor blood was studied.
Object of Research
Red blood cells from preservation of blood of donors (E, which are intended for component transfusion therapy.
Materials and Methods
The upgraded resuspending solution was obtained by treating
saline sodium chloride with standardized magnetite nanoparticles
(ICNB) using the Belousov’s method [7] with complete removal
of NPs from the solution. Magnetite NPs synthesized by coprecipitation
method. Basic physical and chemical properties of
ICNB:
A. Concentration of the colloidal solution of magnetite NPs
in physiology solution of NaCl is 0.0225%.
B. Theoretical osmolality of colloid solution is 500mosmol/l
C. Size of magnetite nanoparticles is 6-12nm;
D. Total area of surface magnetite of nanoparticles Ss = 800-
1200m2/g;
E. Magnetization of saturation Is = 2.15кА/m;
F. ζ - potential = - 19mV.
Erythrocyte-Containing Components (ECC) obtained according
to the standard method from the peripheral blood of adult healthy
donors prepared on the preservative CPDA-1 were studied (n = 20,
160 studies). To study the effect of a nanotechnologically upgraded
0.9% NaCl solution, the component containing red blood cells was
divided into 2 equal aliquots. Red blood cells from the first aliquot
(A1) were resuspended in a modified saline solution of sodium
chloride (a+). As a control, a second aliquot (A2) was used, in which
a standard saline solution of sodium chloride (bK) was used to
resuspend red blood cells. Resuspended red blood cells were stored
under hypothermia (temperature 2-6 °C) and examined for 2, 9, 16,
23, 30, 37, 44, 49-per day from the moment the beginning of blood
preservation (then - sequentially stage I, II, III, IV, V, VI, VII, VIII).
Evaluation of the activity of Lipid Peroxidation (POL) processes
was carried out by the spectrophotometric method’s Volchegorsky
[20] in the modification [21], which makes it possible to differentiate
the products of peroxidation of phospholipids (ph) extracted in the isopropanol phase and neutral lipids (n) extracted in the heptane
phase. The optical density of each phase was measured on a Helios
spectrophotometer at a wavelength of () = 220nm, reflecting the
content of Isolated Double Bonds (IDB) in the extracted unsaturated
fatty acid substrates. The concentration of Diene Conjugates (DC)
was observed at = 232nm; Triene Conjugates (TC) - at IC =
268nm Oxidiene Conjugates (ODC) - at = 278nm and end Schiff
bases products (SB) - at = 400nm. The control was heptane I
and the isopropanol phase of water. Catalase (AK) activity [22],
glucose content [23], and levels of Red Blood Cells Resistance to
Peroxide (RBCR) were also determined [24]. Statistical verification
of the obtained data was carried out using computer programs
STATISTICA 6.1 (StatSoft, USA) [25-27].
Research and Result
Table 1 shows data on indicators that had a statistically significant difference at the first stage of the study between the control and experimental groups. The data in Table 2 show that at Stage III of the study, according to the Student’s t-test, the main group (a+) has a statistically lower level of DCph compared to the control (bC) group - by 1.6 times (P = 0.011354), TCph - by 1.4 (p = 0.015954) and ODCph - by 1.5 times (P = 0.012931). Table 2 also shows that the processes of peroxidation of neutral lipids in the experimental group compared to the control group were 1.3 times lower, and the concentrations of DCn (P = 0.036659) and TCn - 2.2 times (P = 0.021825). Subsequent storage of ECC (stages IV and V of the study) showed intergroup differences in the formation of lipid peroxidation end-products (Table 3 & 4).
Table 1: The POL, AC and RBCR indicators in red blood cell samples containing the component of the experimental (a+) and control (bC) groups, which at the stage I showed a statistically significant intergroup difference.
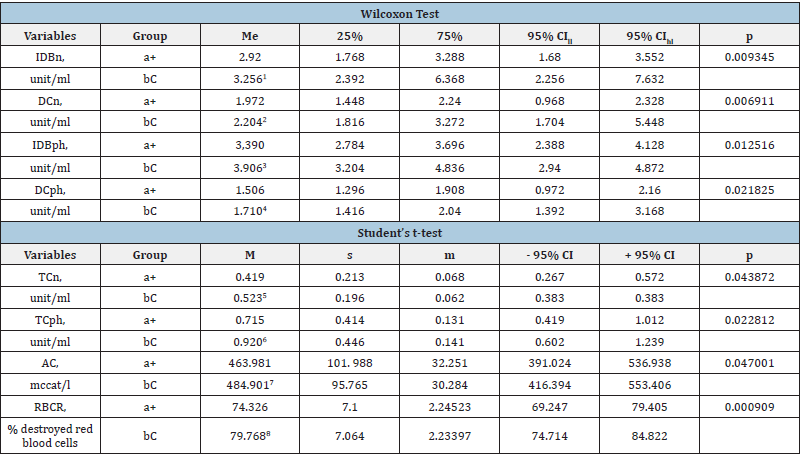
Notes: M - the arithmetic mean; s - the root - mean - square (standard) deviation; m - the standard error of the arithmetic mean; CI - the confidence interval; me - the median; 25% and 75% are percentiles; CI - the confidence interval; CIll - the lower limit of CI; CIhl - the upper limit of CI; IDB DC, TC - respectively isolated double bonds, diene and triene conjugates in neutral lipid peroxidation (n) phospholipids (ph);); 1-8 - statistically significant difference in indicators between (a+) and (bC) groups.
Table 2: Lipoperoxidation (Pol) data in red blood cell samples containing the experimental (a+) and control (bC) groups, which at Stage III showed a statistically significant intergroup difference.
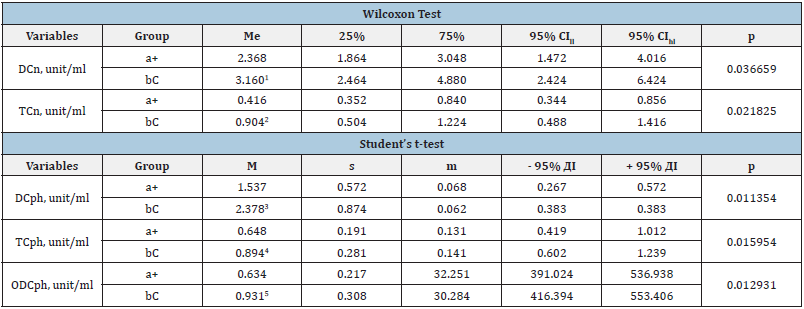
Notes: M - the arithmetic mean; s - the root - mean - square (standard) deviation; m - the standard error of the arithmetic mean; CI - the confidence interval; me - the median; 25% and 75% are percentiles; CI - the confidence interval; CIll - the lower limit of CI; CIhl - the upper limit of CI; IDB DC, TC - respectively isolated double bonds, diene and triene conjugates in neutral lipid peroxidation (n) phospholipids (ph); 1-5 - statistically significant difference in indicators between (a+) and (bC) groups.
Table 3: Lipoperoxidation indicators in the ECC of the experimental (a+) and control (bC) groups, which at Stage IV showed a statistically significant intergroup difference.
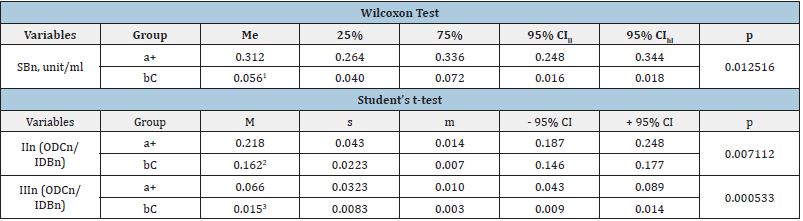
Notes: M - the arithmetic mean; s - the root - mean - square (standard) deviation; m - the standard error of the arithmetic mean; CI - the confidence interval; me - the median; 25% and 75% are percentiles; CI - the confidence interval; CIll - the lower limit of CI; CIhl - the upper limit of CI; SB, DC, TC - respectively isolated double bonds, diene and triene conjugates in neutral lipid peroxidation (n) phospholipids (ph); 1-3 - statistically significant difference in indicators between (a+) and (bC) groups.
Table 4: Lipoperoxidation indicators in red blood cell samples containing a component of the experimental (a+) and control (bC) groups, which at Stage V showed a statistically significant intergroup difference (Student’s t-test).
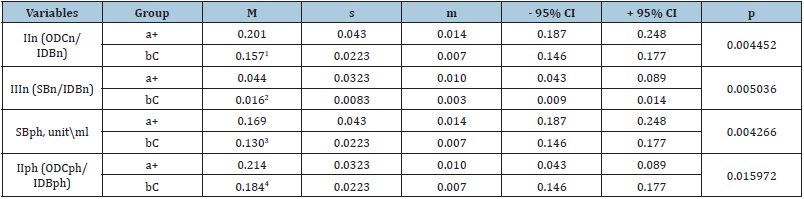
Notes: M - arithmetic mean; s - root– mean – square (standard) deviation; m - standard error of the arithmetic mean; CI - confidence interval; II and III - respectively oxidation indices of secondary and final molecular products (II-according to the ODC/IDB ratio and III-SB/IDB) during peroxidation of neutral lipids (n) and phospholipids (ph); IDB, ODC, SB - respectively isolated double bonds, oxodiene conjugates and bases of Schiff products; 1-4 - statistically significant difference in lipoperoxidation rate between a+ and bC groups.
Table 3 & 4 show that at stages IV and V of the study, there is a statistically significant difference in the content of BSn, BSph, as well as in the oxidation rates of secondary (IIn - ODCn/IDBn ratio, IIph (ODCph/IDBph) and final (IIIn - BSn/IDBn ratio) molecular LPo products. Further statistical analysis of the data (starting from Stage VI and ending with Stage VIII) did not reveal significant differences between POL, AC and RBCR indicators between the samples of the experimental and control groups. Thus, the results of the conducted studies showed that the use of experimental NUS for resuspending improves the quality properties of preservation of blood by reducing the concentration of primary and secondary molecular products of POL during the first 15 days of canning in hypothermia. The applied solution significantly reduces formation bases of Schiff products at stages IV-V of the study and, as a result, reduces the concentration of POL final products. There was not statistically significant difference between the studied indicators between the experimental and control groups starting from Stage VI and up to Stage VIII inclusive. Statistically significant intergroup differences in AC and RBCR indicators were found only at the stage I. Changes in POL values correlate with the storage time of ECC samples.
Discussion
It is known that the potential of nanoparticles for medical
Biotechnologies lies primarily in their use as diagnostic tools,
drug delivery, and therapy [28-30]. Modification of NPs surfaces
significantly affects the physical, chemical, and biological nature
of entire molecules in biological systems [31]. The main risk factor
for the use of medical nanotechnologies is their possible toxicity,
potential ability to damage biomembranes and affect the function
of biomolecules, including cellular organelles. The mechanism of
action of NPs is associated with the formation of free radicals in the
presence of nanoobjects [32,33]. The NUS used in this study does
not contain magnetite NPs due to the use of a special technology for
their pre-removal after exposure to solution molecules [7]. Based
on the results of studies of the effect of two resuspending solutions
on oxidative homeostasis indicators, it was found that at the first
three stages (15 days of storage),the advantage was on the side of
experimental NUS due to a statistically significant decrease in the
level of primary and secondary POL products, including IDBn, DCn,
TCn, IDBph, DCph, TCph, relative to the data recorded in the control
group. In addition, the intergroup difference in the first stages of
the study was confirmed by other methods (according to AC and
RBCR data).
In addition, resuspending in NUS inhibited the shear reaction
during neutral lipid peroxidation in the late stages of storage.
Thus, there is a statistically significant intergroup difference in
the SBn content (p = 0.012516 according to the Wilcoxon test)
and the oxidation rates of secondary and final POL molecular
products (respectively: ODCn/ IDBn ratio, p = 0.007112 and SBn/
IDBn, p = 0.000533 according to the Student’s t-test). Such results
were obtained due to the effect of NUS on the metabolism of red
blood cells for resuspending when the structure of liquid medium
molecules changes under the influence of NPs magnetite [34].
The NUS used in the study is as simple as possible in composition
and, therefore, is available for technological reproduction in
Blood Service Institutions. Due to the complete removal of NPs,
the drug meets the requirements for such decisions by regulatory
authorities.
Conclusion
The saline sodium chloride solution which nanotechnologically upgraded by standardized biocompatible forms of NPs magnetite, has a positive effect on the ECC of donor blood when used as a resuspending agent. The advantage of exposure to this solution was a decrease in the intensity of POL reactions, an increase in AC and RBCR on the first or second day after resuspending the erythrocytes were compared with a standard solution.
References
- Smit SC, Taswell HF (1984) eBook: Quality assurance in blood banking and its clinical impact. Martinus Nijhoff Publishers, Boston, USA.
- Smit S, Das PC, Loghem, van JJ (1982) Blood Transfusion and Problems of Bleeding.
- nanolab.com.ua
- Patent of Ukraine №14817А UA A61N2/00 Method of production of a magnetic liquid for transport and retention of medicines in organism.
- Patent Agency of Ukraine No. 30538А UA A 23L 1/304 Therapeutic and preventive product MICROMAGE-B.
- Patent of Ukraine №24322А UA A61N2/00 The sorbent for extracorporal detoxycation of biological liquids.
- Patent of Ukraine №24183А UA A61N2/00 The method extracorporal detoxycation of biological liquids.
- Belousov AN (2011) The use of magnetite nanoparticles in applied medicine. International Journal of Nano Dimension 1(5).
- Belousov AN (2021) Investigation of the influence nanoparticles of magnetite-controlled sorbent (MCS-B) on the functional activity of erythrocytes. Prospects Medicine and Biology, pp. 94-97
- Belousov AN (2008) Opening the mechanisms of cell regulation by nanotechnology preparations. 7th International Conference on the Scientific and Clinical Applications of Magnetic Carriers Vancouver, Canada, pp. 234-235.
- Patent of Ukraine №31309А UA A61N2/00 Method of treatment diseases which connected with blood circulation disturbance.
- Patent of Ukraine №42123А UA A61N2/00 Method of treatment diseases which connected with endocrine disturbance / A.N. Belousov № 98084233. Decl. 04.08.98; Publ. 15.10.01.
- Patent of Ukraine 42132А UA A61N2/00 Method of treatment digestion system diseases.
- Belousov AN, Voyda Yu (2005) Study of the reactions of microorganisms in response to the magnetite nanoparticles. OALib J 2(4): 1-5.
- Belousov AN (2017) New prospects application of magnetite nanoparticles for the diagnosis of malignant tumors in MRI investigation. J Cell Mol Biol 2: 1-4.
- Belousov AN (2014) The role of magnetite nanoparticles (ICNB) in discovery new factor which influence on permeability of erythrocytes and eryptosis. Journal Nanoscience and Nanotechnology Research 2(1): 8-11.
- Belousov AN (2009) Spectrum of application magnetite nanopaticles in medicine. Nanotech 2(3): 154-157.
- Belousov AN (2013) Ultrastructure of hepatic cells in rabbits after injection of nanoparticles MCS-B. Journal Nanotechnology Bio Sensors, Instruments, Medical, Environment and Energy 3: 258-260.
- Belousov AN (2008) Opening the mechanisms of cell regulation by nanotechnology preparations /7th International Conference on the Scientific and Clinical Applications of Magnetic Carriers - Vancouver, Canada, pp. 234-235.
- Volchegorsky IA, Nalimov AG, Yarovinsky BG, Lifshits RI (1989) Comparison of different approaches to the determination of LP products in heptane-isopropanol blood extracts. Questions of medical chemistry 1: 127-131.
- Anoshyna M, Kalynychenko TO, Gluhen’ka GT (2011) Evaluation of lipid peroxidation in samples of cryopreserved umbilical cord blood. Ukrainian Journal of Hematology and Transfusiology 3: 12-15.
- Balakhovsky SD, Balakhovsky IS (1953) Methods of chemical analysis of blood, Russia.
- Menshikov VV (1987) Laboratory research methods in the clinic: handbook, Russia, p. 232.
- Grigorovich NA, Mavrichev AS, Bychkov G, Lysenko AA, Сopyright сertificate 1704083 RF, MKI 01 33/50. A method for assessing the peroxide resistance of erythrocytes, Russia.
- Rebrova O (2006) Statistical analysis of medical data. Application of the STATISTIS application software package. Moscow, Mediasphere, Russia, p. 312.
- Petri A, Sabin K (2015) Visual medical statistics: textbook. Manual trans, Russia, p. 216.
- Grzhibovsky AM, Ivanov SI, Gorbatova MA (2016) Comparison of quantitative data of three or more paired samples using Statistica and SPSS software: parametric and nonparametric criteria. Science and Healthcare 5: 5-29.
- Rouchota M, Adamiano A, Iafisco M, Fragogeorgi E, Pilatis I, et al. (2021) Optimization of In Vivo studies by combining planar dynamic and tomographic imaging: Workflow evaluation on a superparamagnetic nanoparticles system. Mol Imaging.
- Ribarič S (2021) Nanotechnology therapy for alzheimer's disease memory impairment attenuation. Int J Mol Sci 22(3): 1102.
- Javan Nikkhah S, Thompson D. (2021) Molecular modelling guided modulation of molecular shape and charge for design of smart self-assembled polymeric drug transporters. Pharmaceutics 13(2):141.
- Kang H, Mintri S, Menon AV, Lee HY, Choi HS, et al. (2015) Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 7(45): 18848-18862.
- Abaeva LF, Shumsky VI, Petritskaya EN, Rogatkin DA, Lubchenko PN (2010) Nanoparticles and nanotechnologies today and beyond. Almanac of Clinical Medicine 22: 10-16.
- Nguyen KC, Rippstein P, Tayabali AF, Willmore WG (2015) Mitochondrial toxicity of cadmium telluride quantum dot nanoparticles in mammalian hepatocytes. Toxicol Sci 146(1): 31-42.
- Belousov AN (2013) Application magnetite of nanoparticles (ICNB Preparation) as magnetically resonant contrasting means during visualization of tumours. Clean Technology and Sustainable Industries Organization, pp. 379-381.
© 2021 Andrey Belousov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






