- Submissions

Full Text
Associative Journal of Health Sciences
Determining the Concentrations of Lithium and Calcium in Canned Tuna Fish Produced by the Factories at Khuzestan province, Iran
Mona Daraei1*, Heibatullah Kalantari2 and Zahra Nazari Khoragani3
1Arvand International Division, Ahvaz Jundishapur University of Medical Sciences, Iran
2Department of Toxicology, Ahvaz Jundishapur University of Medical Sciences, Iran
3Nanotechnology Research Center, Ahvaz Jundishapur University of Medical Sciences, Iran
*Corresponding author: Mona Daraei, Arvand International Division, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, IR Iran
Submission: July 20, 2021;Published: October 13, 2021

ISSN:2690-9707 Volume1 Issue4
Abstract
Backgrounds and aims: Nutrition is the main way to receive the elements that human body needs. Lithium (Li) and Calcium (Ca) are two elements, which have important roles in human body. There are a few studies about the amounts of calcium and lithium in canned tuna fish. The aim of this study was to determine the concentrations of calcium and lithium in canned tuna fishes.
Materials and methods: To this aim, 150 samples of canned tuna fishes from two well-known brands (Majid and Poolak), marketed in Khuzestan province of Iran, were obtained. Li contents of the samples were measured by Atomic Absorption Spectrometry (AAS) with atomization in graphite furnace. Ca contents of the samples were measured by AAS using nitrous oxide-acetylene flame.
Result: The mean concentrations of calcium in canned tuna fish of Majid and Poolak brands were 448.47 and 398.22mg/kg, respectively. Additionally, the mean concentrations of calcium in canned tuna fish of Majid and Poolak brands were 38.42 and 39.67mg/kg, respectively. The mean concentration of calcium in the investigated canned tuna samples fish was lower than Provisional Tolerable Intake (PTI) per day (1000ppm) set by World Health Organization (WHO) and Environmental Protection Agency (EPA). However, the mean concentration of lithium in the studied canned tuna fish samples was higher than PTI/day (0.650- 3.1ppm) Set by EPA.
Conclusion: The results of current study showed that the marine food industries need a more precise and serious monitoring by public health organizations. Additionally, protecting the marine environment from pollutions is an obligation that needs the attention of related organizations and authorities.
Keywords: Lithium; Calcium; Canned tuna fish; Atomic Absorption spectrometry
Introduction
Consuming healthy natural foods has a great importance for the entire society. In this
regard, receiving sufficient amounts of trace elements and avoiding the excess amounts of
these elements is an important factor, which should be carefully monitored. Seafood products
are important sources of the trace elements [1]. Due to the release of toxic chemicals from
point and non-point pollution sources into surface waters, rivers, lakes and finally into seas,
marine animals are affected. As a result, nutritionists are concerned about potential health
threats related to consumption of the marine animals as seafood. Therefore, it is necessary to
monitor the pollution levels of the marine environment and seafood.
Nowadays, using Lithium (Li) is rapidly increasing due to its importance as a vital strategic
resource for national economy and protection [2]. Li is an element which appears rather in
small amounts both in the environment and in the human tissues or body fluids. Li plays key roles in many important vital functions. Li possesses a wide variety
of biochemical functions. For example, it settles in the structure of
several enzymes, hormones, vitamins, and growth factors [3,4]. Li
is found in variable amounts in foods. Main food sources of Li are
grains and vegetables; in some geographical areas, the drinking
water also provides significant amounts of lithium. Thus, the
human dietary lithium intakes depend on the geographical location
and the type of foods consumed [5]. Many studies consider Li as
an essential element; so that, 1000μg/day of it has been suggested
as provisional RDA for a 70kg adult person [5]. Receiving excess
amounts of lithium via foods may induce lithium toxicity, which
include several serious clinical manifestations (e.g., Cardiovascular
manifestations and permanent neurological deficits) [6].
The Calcium (Ca) function and its role in the body is well known
and expansively defined in literature. It is the most abundant
mineral within the body, of which almost 99% happens in the
bones and teeth as deposits of calcium phosphates. The remaining
1% in the soft tissue and body fluids has very important regulatory
functions [7]. The calcium ion (Ca+2), released from intracellular
stores, controls numerous cellular procedures, including cardiac
and skeletal muscle contraction, synaptic transmission, and
metabolism [8]. Older men and women are recommended to
take at least 1000-1200mg/day of calcium for maintaining bone
health and prevention of fractures. The average intake in the diet
in Western countries is 700-900mg/day, and lower in Asia and
Africa. Accordingly, old people need to take calcium supplements
to meet these recommendations. The guidelines for calcium
intake have been widely implemented in Western countries. So
that, more than 30-50% of old women take calcium supplements.
However, several clinical trials reported that calcium supplements
at doses higher than 2000mg/day lead to adverse effects, including
cardiovascular events, kidney stones, and hospital admissions for
acute gastrointestinal symptoms. Thus, old people are encouraged
to improve their bone health by increasing their calcium intake
via food rather than taking supplements. This advice assumes
that increasing dietary calcium intake to the recommended level
of >1200mg/day prevents fractures without causing the adverse
effects of calcium supplements [9].
Recent studies indicate the heavy metals pollution of seas
and seafood in many regions of the world, including Iran [10-
12]. The polluted sea food may lead to serious health problems
in the people who consume them. Therefore, it is important to
prevent and monitor the pollution in seafood products. According
to the literature above, the present study aimed to evaluate the
concentrations of two elements, calcium and lithium, in canned tuna
fish as an important food source of local population at Khuzestan
province of Iran.
Materials and Methods
Materials and preparing standard solutions
Chemicals were purchased from Chem Lab and Merck Companies. All the glassware and plastic ware were put in nitric acid (0.1 molar) for 24 hours, and then washed twice with deionized water. Stock standard solutions for Li and Ca were 1000mg/lit. Lanthanum (III) nitrate and ammonium nitrate solutions (1%w/v) were used as modifiers for Ca and Li respectively, to eliminate the effect of disturbing ions. The lithium standard solutions were prepared in five concentrations (40, 60, 80, 100, 120μg/lit) by diluting the stock standard solution in nitric acid (0.1 molar). The Calcium standard solutions were prepared in six concentrations (0.1, 0.2, 0.5, 1, 3, 5μg/lit) by diluting the stock standard solution in Lanthanum (III) nitrate (1%w/v).
Preparation of samples and standard samples
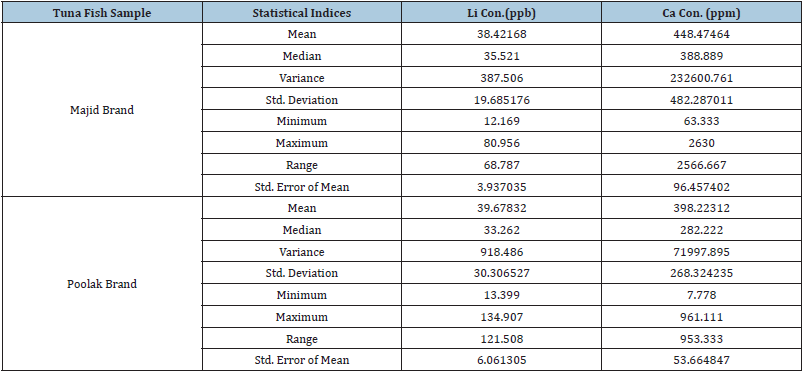
Table 1: Li and Ca content in canned tuna fish, its mean values per 50g of canned tuna fish and their range.
150 samples of canned tuna fish (Majid and Poolak brands) were collected from the local markets in Khuzestan Province, Iran. All of the collected canned tuna fish samples were mixed (each brand separately) and a representative mass (50g) was taken and placed in a porcelain crucible for further steps of examination. Samples were transferred into the electrical furnace with controlled temperature (450 °C) for 24h to turn them into their ash. Then the ash samples in the crucible were carefully preserved with 100mL of concentrated HNO3. By this procedure, the ash samples became readily soluble in water. Sample solutions were stored in washed and dried plastic bottles. The sample solutions were pulverized into spray in the nebulizer and atomized into the nitrous-acetylene flame of the spectrometer. The air oxygen was used as oxidant Table 1 & 2.
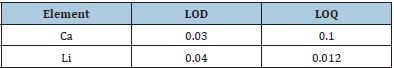
Table 2: Limits of Detection (LOD), Limit of Quantification (LOQ) for Li and Ca (mg/kg).
Instrumental analysis
The content of Li in the samples was determined by Atomic Absorption Spectrometry (AAS) using Varian AA240FS spectrometer (Palo Alto, California, United States). Measurable analysis was performed in the graphite furnace, GTA 120 Graphite Tube Atomizer (Palo Alto, California, United States). Measurements were performed in the pyrolytically coated tubes under an atmosphere of the inert gas Argon (Ar) in the following conditions: The wavelength was 670.8nm. The temperature of pyrolysis and atomization were 800 °C and 2450 °C, respectively. The concentrations used for drawing the calibration curve were 40.0, 60.0, 80.0, 100.0, 120.0μg/L. the current within the Hollow- Cathode Lamp (HCL) was 5mA. The slit width was 0.5 nm. Singleelement hollow analytic cathode lamps were used as the radiation source. The determination parameters of Lithium by AAS method is presented in Table 3.
Table 3: The parameters for the determination of Lithium by the AAS Method.

The content of Ca in the sample was determined by Flame Atomic Absorption Spectrometry (FAAS). Operating parameters were set as recommended by the manufacturer manual. The spectrometer and the flame conditions were adjusted to yield optimum precision and sensitivity, to maximize absorbance signals and to minimize backgrounds set at 422.7nm wavelengths for Ca. the current within the Hollow-Cathode Lamp (HCL) was 10mA and the slit width was 0.5nm. To establish the precision of the explained and applied analytical procedure, an appropriate validation was performed. The Limits of Detection (LOD) were used to identify any cross corruption or memory effects from sample preparation (calculated from: 3.3 × SD/a, where: SD standard deviation for blanks; the slope of calibration curve). Thus, each test run included one reagent blank control. The LODs of Ca and Li, given that the Limit of Quantification (LOQ), correspond to Table 2. Unfortunately, there are no suitable certified reference materials for Li content in biological media. Its quantity is reported only in a few materials. The instrumental drift was verified in each 100 samples (plus one at the end) by the control of the sensitivity of one calibration standard.
Statistical analyses
Statistical analyses were performed using SPSS software version20. Results were expressed in the form of mathematics mean (x) ±SD. Analysis of variance )T test-One Sample) was used to compare results in groups of canned tuna fish (Mann-Whitney test, p < 0.05) and ( Independent Sample T Test) for correlation analysis between variables.
Result
As shown in Table 1, the results of the study showed that mean ±SD concentrations of Li for Majid and Poolak brands were 38.4±19.6ppm and 39.7±30.3ppm, respectively. Additionally, the mean±SD concentrations of Ca for Majid and Poolak brands were 448.5±448.3ppm and 398.2±268.3ppm, respectively. Other statistical indices are shown in Table 1.
Discussion
Current study showed that the amount of Li in canned tuna
fish was less than 40ppb (<40μg/kg). this result showed that
consuming the canned tuna fish as a meal does not increase the
Li intake to a level higher than the recommended daily intake of
lithium (14.3μg/kg body weight) for an adult person [13]. The
Li content of the canned tuna fish was also comparable with the
Li contents of other food groups [14]. This finding matches the
common sense since marine foods are not usually among the food
sources with the highest Li content. Indeed, cereals, vegetables, and
dairy products contain the highest levels of Li. Fortunately, current
study showed that the canned tuna fish is a safe food, regarding the
Li content and it is not affected by probable metal pollutions of the
Persian Gulf (Table 4).
The results of current study indicated that the amount of Ca
in canned tuna fish was less than 450ppm (<450mg/kg). This
amount was consistent with the previous studies, which reported
comparable amounts of calcium in tuna fish [15,16]. The findings
indicated that tuna fish is a rich dietary source of Ca, which can
supply human daily needs, together with other food groups [17].
In this study, the levels of Li and Ca, in samples of canned tuna fish in local brands of Khuzestan province was reported. These results
showed that there is no risk in canned tuna fish with respect to the
concentrations of Li and Ca and their recommended daily intake
[18]. These results may provide useful information for measuring
of Li and Ca intake from this source. In recent years, fish and its
products have become more attractive to human societies, because
of their health benefits, compared to the other types of meat. Due
to the growing trend of consumption of marine products, especially
fish and canned tuna fish, the quality control of these materials
is essential for maintaining and improving the health of human
communities. Therefore, monitoring these products is important
with respect to positive and negative effects of Li and Ca on human
health.
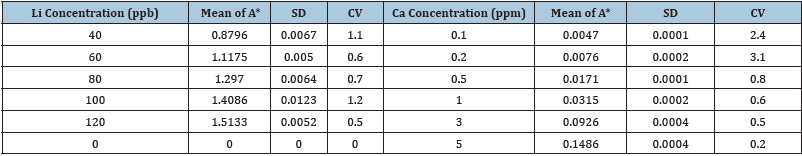
Table 4: Recovery study of Li and Ca in canned tuna fish.
Conclusion
The canned tuna fish produced by Khuzestan factories were appropriate food sources for Li and Ca dietary intake, while avoiding the toxic effects of receiving excess amounts of the elements. It is also strongly recommended that inclusive and periodic monitoring of the trace and essential metals in the canned tuna fish must be done to ensure the safety and health of the consumers. Comparison of the results obtained in this study with the values reported in literature showed that the consumption of the canned tuna fish provides higher amount of Li and significant amount of calcium. It was, however, recommended that inclusive and periodic monitoring of the trace and essential metals in the canned tuna fish must continue to ensure the safety of the health of the consumers.
Acknowledgment
This study was supported by Arvand International Division, Ahvaz Jundishapur University of Medical Sciences Ahvaz, Iran.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
References
- Guérin T, Chekri R, Vastel C, Sirot V, Volatier JL, et al. (2011) Determination of 20 trace elements in fish and other seafood from the French market. Food Chemistry 127(3): 934-942.
- Liu L, Zhang H, Zhang Y, Cao D, Zhao X (2015) Lithium extraction from seawater by manganese oxide ion sieve MnO2 0.5 H2O. Colloids and Surfaces A: Physicochemical and Engineering Aspects 468: 280-284.
- Shorter E (2009) The history of lithium therapy. Bipolar Disorders 11(s2): 4-9.
- Shaldubina A, Agam G, Belmaker RH (2001) The mechanism of lithium action: state of the art, ten years later. Progress in Neuro-Psychopharmacology and Biological Psychiatry 25(4): 855-866.
- Schrauzer GN (2002) Lithium: occurrence, dietary intakes, nutritional essentiality. Journal of the American College of Nutrition 21(1): 14-21.
- Zyoud SeH, Waring WS, Sweileh WM, Al Jabi SW (2017) Global research trends in lithium toxicity from 1913 to 2015: A bibliometric analysis. Basic Clin Pharmacol Toxicol 121(1): 67-73.
- Nabrzyski M, Gajewska R (2002) Content of strontium, lithium and calcium in selected milk products and in some marine smoked fish. Die Nahrung 46(3): 204-208.
- Santulli G, Marks A (2015) Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Current Molecular Pharmacology 8(2): 206-222.
- Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, et al. (2015) Calcium intake and risk of fracture: systematic review. BMJ 351: h4580.
- Sattari M, Vajargah MF, Bibak M, Bakhshalizadeh S (2020) Relationship between trace element content in the brain of bony fish species and their food items in the southwest of the caspian sea due to anthropogenic activities. Avicenna J Environ Health Eng 7(2): 78-85.
- Supriya RA, Sureshkannan S, Porteen K, Masilamoni BS, Ronald K, et al. (2020) Investigation of heavy metal concentrations in sea food from three selected landing centers of Chennai coast. International Journal of Chemical Studies 8(4): 08-14.
- Bat L, Arici E (2018) Heavy Metal Levels in Fish, Molluscs, and Crustacea From Turkish Seas and Potential Risk of Human Health. In: Holban AM, Grumezescu AM, editors. Food Quality: Balancing Health and Disease: Academic Press; 2018. p. 159-96.
- Aral H, Vecchio SA (2008) Toxicity of lithium to humans and the environment-A literature review. Ecotoxicology and Environmental Safety 70(3): 349-356.
- Leblanc JC, Guérin T, Noël L, Calamassi TG, Volatier JL, et al. (2005) Dietary exposure estimates of 18 elements from the 1st French Total Diet Study. Food Additives & Contaminants 22(7): 624-41.
- Abbey L, Glover AM, Atikpo MO, Atter A, Toppe J (2017 ) Nutrient content of fish powder from low value fish and fish byproducts 5(3): 374-379.
- Talib A, Hariati AM, Nurhidayati F (2020) The mineral content and vitamin d on bone flour fish yellowfin tuna. Journal of Physics: Conference Series 1517: 012042.
- Uusi RK, Kärkkäinen MUM, Lamberg A (2013) Calcium intake in health maintenance-a systematic review. Food & Nutrition Research 57(1): 21082.
- Fogelholm MJF (2013) New Nordic Nutrition Recommendations are here. Food Nutr Res 3: 57.
© 2021 Mona Daraei. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






